Overview
This article delves into best practices for leveraging Real-World Evidence (RWE) in Brazilian medtech trials, underscoring its increasing significance in regulatory decision-making and patient care. By illustrating how RWE deepens the understanding of device performance in real-world environments, it highlights its role in streamlining regulatory processes and enhancing stakeholder engagement. Ultimately, this leads to improved healthcare outcomes and fosters innovation within the medtech sector.
Introduction
In the ever-evolving landscape of medical technology, the integration of Real-World Evidence (RWE) is revolutionizing how clinical trials are conducted and evaluated. As healthcare demands more efficient and effective solutions, RWE emerges as a powerful tool, providing insights derived from data collected outside traditional clinical settings. This shift not only influences regulatory decisions but also enhances patient outcomes and streamlines the development of innovative medical devices.
With regulatory bodies increasingly recognizing the value of RWE, stakeholders must navigate the complexities of data collection, ethical considerations, and stakeholder engagement to fully leverage its potential. This article explores the critical role of RWE in Medtech trials, particularly in Brazil, highlighting successful applications, challenges, and best practices that can guide companies in their quest for regulatory approval and improved healthcare solutions.
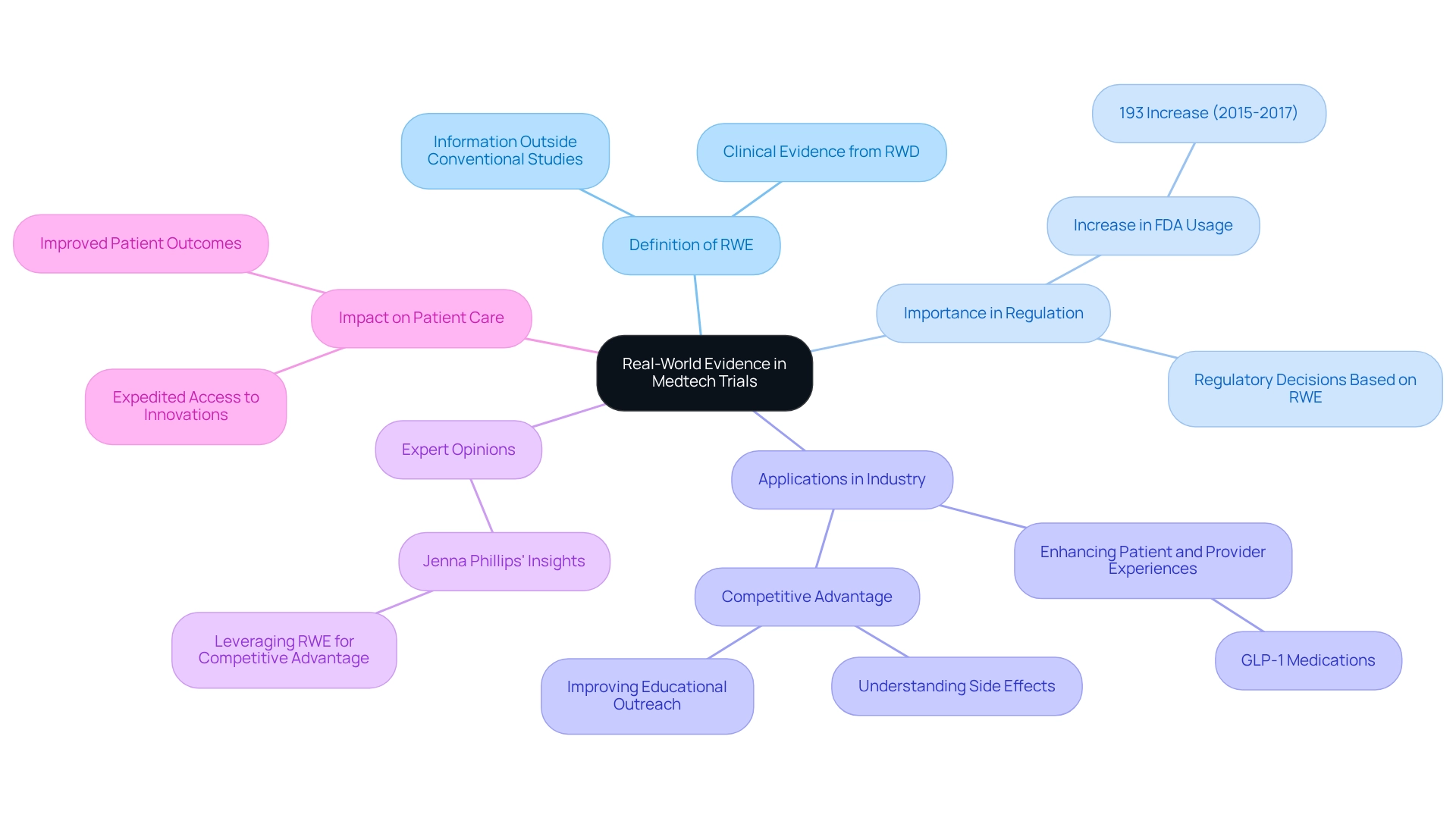
Understanding Real-World Evidence in Medtech Trials
Real-World Evidence (RWE) is defined as clinical evidence obtained from the analysis of Real-World Data (RWD), which encompasses information gathered outside the parameters of conventional clinical studies. In the Medtech landscape, the use of real-world evidence for Brazilian medtech trials is gaining traction for its ability to inform regulatory decisions, improve patient outcomes, and support the development of medical devices. By leveraging RWE, researchers can obtain valuable insights into device performance in real-world settings, offering a more nuanced understanding of their effectiveness and safety.
The importance of RWE in medical device regulation cannot be overstated. Regulatory bodies, including the FDA, have increasingly relied on RWE to make informed decisions, with a notable 193% increase in its use for pre- and post-market regulatory decisions from 2015 to 2017. This trend underscores the growing recognition of RWE as a critical component in demonstrating the value of new technologies to both regulators and payers, ultimately expediting patient access to innovative solutions.
RWE is playing a pivotal role in regulatory decision-making, further solidifying its importance in the Medtech sector. Recent advancements emphasize the effective use of real-world evidence for Brazilian medtech trials in Medtech studies. For instance, pharmaceutical companies are utilizing real-world data to enhance patient and provider experiences, particularly in competitive categories such as GLP-1 medications. By focusing on understanding side effects and improving educational outreach, these companies have gained a competitive edge, fostering better brand equity and patient trust.
In Latin America, bioaccess® exemplifies the integration of real-world evidence for Brazilian medtech trials into trial management, offering comprehensive services that encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Their expertise in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF) positions them as a leader in Medtech research in the region. This not only enhances the regulatory landscape but also contributes to local economies through job creation and healthcare improvements.
Expert opinions further reinforce the significance of real-world evidence for Brazilian medtech trials in the industry. Health and life sciences authority Jenna Phillips states, "Utilizing real-world clinical information in innovative manners can lead to significant alterations in pharmaceutical companies' methods of operation to provide a competitive edge to products that are reaching the market faster and more securely than ever before." This insight emphasizes the transformative potential of RWE in enhancing operational efficiencies and market responsiveness.
In summary, the integration of Real-world evidence for Brazilian medtech trials into medical device development is not just a trend but a transformative approach that enhances regulatory decision-making and patient care. As the Medtech industry continues to evolve, the strategic use of RWE, supported by advancements in large data sets and technology, will be essential for companies like bioaccess® aiming to navigate the complexities of regulatory landscapes and deliver effective medical solutions.
Challenges of Conducting Medtech Trials in Brazil
Conducting Medtech studies in Brazil necessitates navigating a landscape characterized by intricate regulatory requirements and prolonged approval timelines. The Brazilian Health Regulatory Agency (ANVISA) oversees research studies; however, the high volume of submissions often leads to delays, complicating the process for Medtech firms. For instance, the typical approval duration for clinical studies can extend considerably, highlighting the resource limitations faced by ANVISA.
Reports indicate that approval timelines can stretch to 12 months or more, posing a significant obstacle for the timely execution of studies.
Logistical challenges further complicate the execution of experiments. Issues such as transportation difficulties and communication barriers can hinder progress, particularly in remote regions where healthcare infrastructure may be deficient. The density of medical imaging equipment, including CT scanners and MRI units, has been tracked from 2010 to 2021, revealing disparities in access to advanced medical technologies across various regions of Brazil.
Moreover, cultural factors must be taken into account; effective patient recruitment strategies demand a nuanced understanding of local populations to ensure adequate participation rates.
A pertinent illustration of these challenges is evident in Brazil's COVID-19 vaccination campaign. As of September 2023, the country has confronted substantial hurdles, including over 37.8 million infections and 705,000 deaths. The campaign, which initially relied on imported vaccines, subsequently incorporated local production, underscoring the necessity for robust partnerships between public laboratories and international institutions to surmount regulatory and logistical obstacles.
As Benny Spiewak, a partner at ZCBS - Zancaner Costa, Bastos e Spiewak Advogados, aptly remarked, "In the end, it will be worthwhile."
Understanding these complex challenges is vital for leveraging Real-World Evidence (RWE) in Brazilian Medtech studies. By grasping the regulatory environment and cultural dynamics, companies can formulate targeted strategies that enhance testing efficiency and effectiveness. bioaccess® provides comprehensive trial management services, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial setup
- Import permits
- Nationalization of investigational devices
- Project management
- Reporting on serious and non-serious adverse events
With over 20 years of expertise in Medtech, bioaccess® is well-prepared to navigate these challenges, ensuring the successful advancement of innovative medical technologies in Brazil. Furthermore, the impact of Medtech clinical studies on local economies, such as job creation and healthcare improvement, emphasizes the significance of international collaboration in promoting global health advancement.
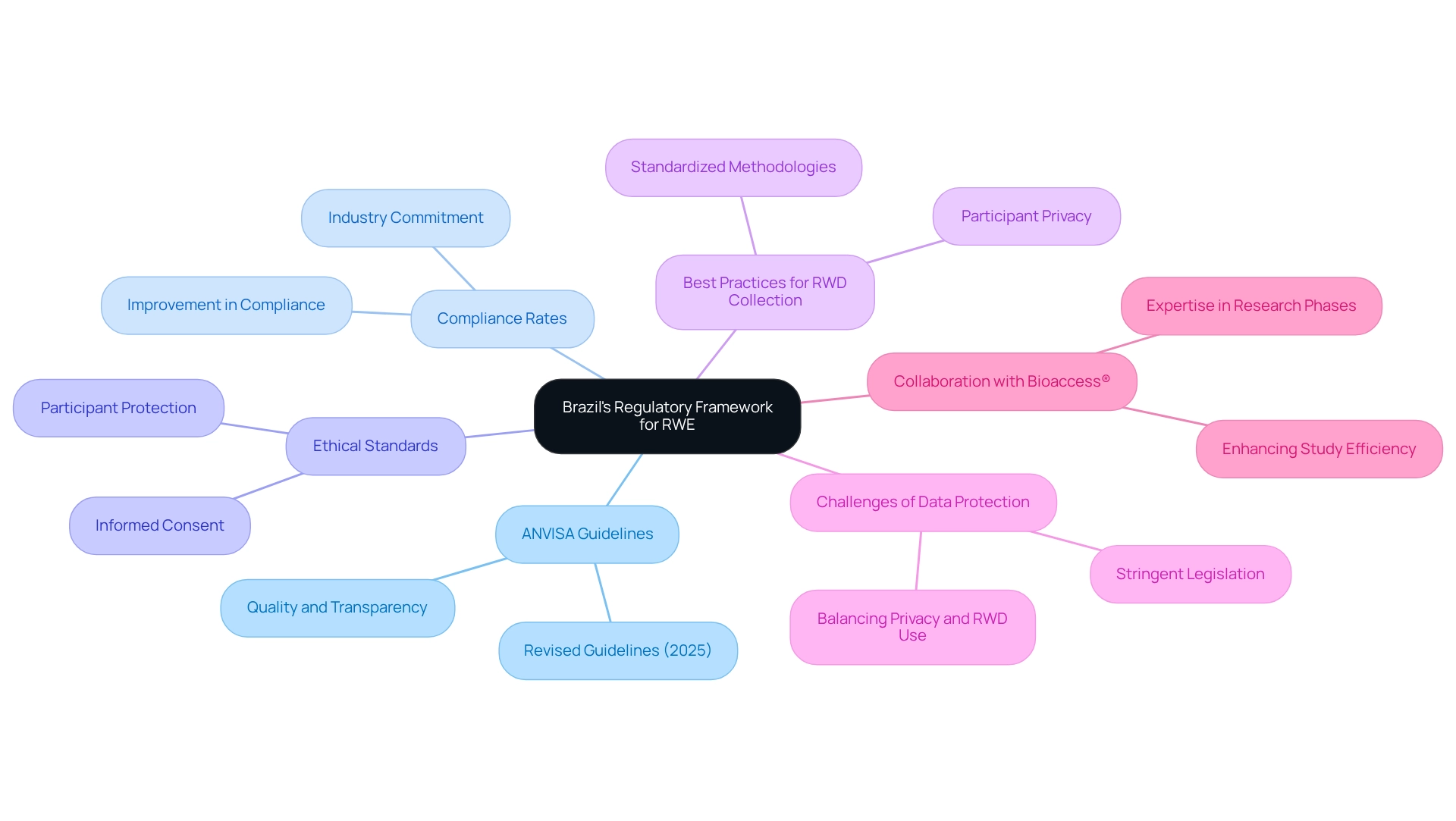
Navigating Brazil's Regulatory Framework for RWE
Navigating Brazil's regulatory framework for Real-World Evidence (RWE) requires a comprehensive understanding of the guidelines established by ANVISA for Brazilian medtech trials. In 2025, ANVISA issued revised guidelines that detail best practices for the gathering and examination of Real-World Information (RWI), emphasizing the critical importance of quality, transparency, and ethical considerations. Notably, the number of regulatory frameworks and guidance documents for RWE has been increasing globally, reflecting a broader trend in the industry.
Compliance rates with ANVISA regulations in Medtech trials have shown significant improvement, underscoring the industry's commitment to adhering to these standards. Medtech companies must ensure that their studies involving real-world evidence for Brazilian medtech trials align with ANVISA's regulations to facilitate acceptance by regulatory bodies. This encompasses following ethical standards, obtaining informed consent from participants, and implementing robust protection measures. Regulatory experts emphasize that aligning RWE strategies with these requirements not only enhances the credibility of the evidence presented but also significantly increases the likelihood of successful product approvals.
As Sean Hennessy from the University of Pennsylvania notes, "The authors declare no conflicts of interest," highlighting the importance of transparency in these discussions. Best practices for collecting RWD in Brazil involve meticulous planning and execution. Companies should focus on establishing clear protocols for information collection that comply with ANVISA's latest guidelines. This involves employing standardized methodologies and ensuring that information is gathered in a way that respects individual privacy rights while still serving the public interest.
A recent case study titled "Privacy and Data Protection in RWD" highlighted the challenges posed by stringent data protection legislation, which, while essential for safeguarding citizens' rights, can complicate the use of RWD in medical research. The report advocates for a balanced approach that provides clear guidelines to navigate these complexities, ensuring robust protections for individuals while facilitating effective RWD utilization.
By integrating these best practices and insights into their RWE strategies, Medtech companies can successfully navigate Brazil's regulatory landscape, ultimately advancing their innovations and contributing to improved healthcare outcomes with real-world evidence for Brazilian medtech trials. Moreover, collaborating with bioaccess®, a prominent Contract Research Organization, can enhance the efficiency and effectiveness of studies in Latin America, leveraging their expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Research.
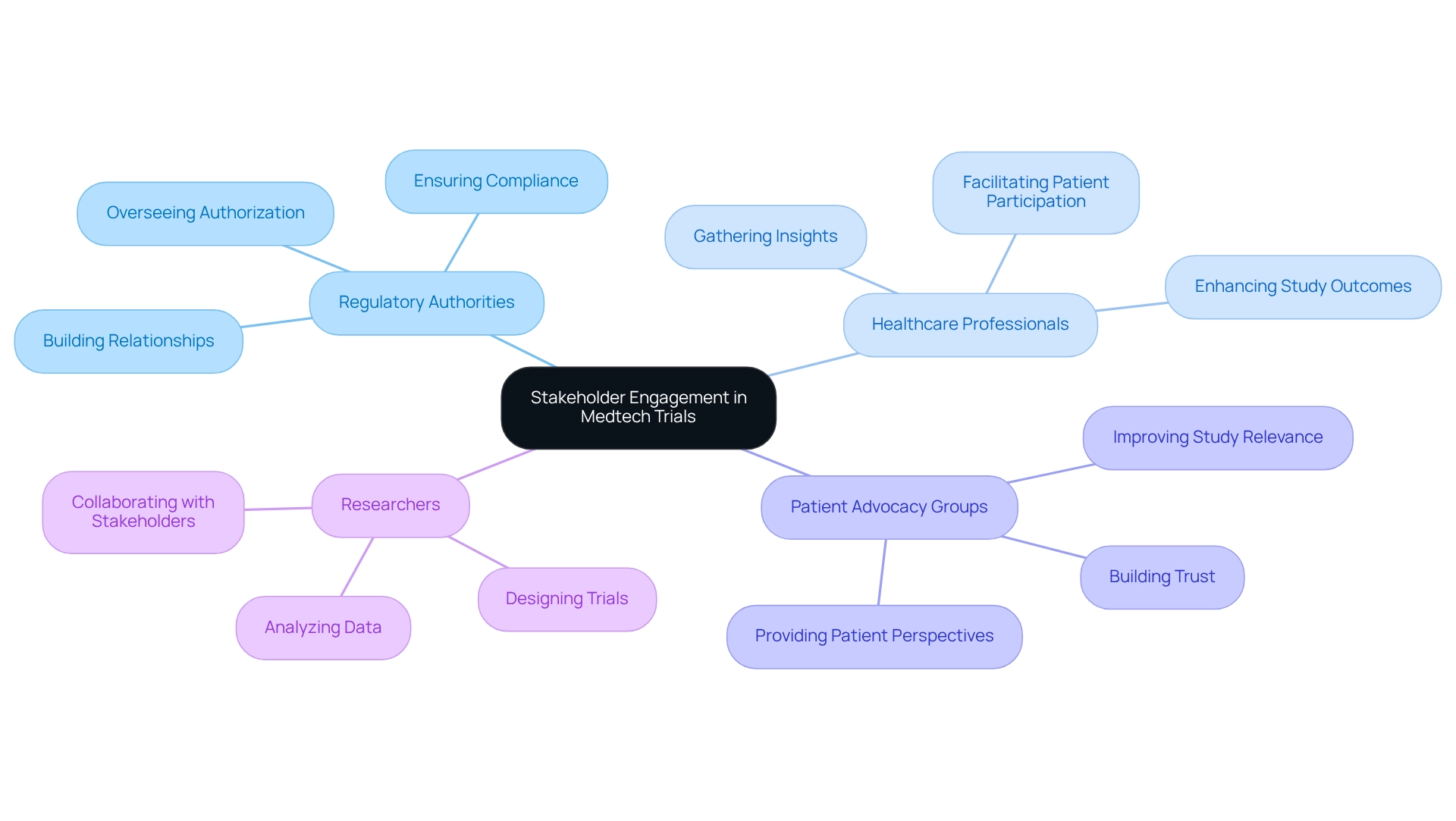
Engaging Stakeholders for Effective RWE Utilization
Involving stakeholders is crucial for effectively utilizing real-world evidence in Brazilian medtech trials. This engagement encompasses building robust relationships with regulatory authorities, healthcare professionals, and patient advocacy groups. Engaging these stakeholders early in the trial design process enables researchers to collect essential insights into patient needs and preferences, significantly enhancing the relevance and impact of medical studies.
At bioaccess, our comprehensive trial management services encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Transparent communication regarding the objectives and benefits of real-world evidence for Brazilian medtech trials fosters trust and encourages patient participation. A recent study highlighted the significance of stakeholder involvement, indicating that participants recognized various groups to engage in upcoming research, with differing levels of consensus across regions.
This underscores the necessity for customized engagement approaches that align with local contexts. Joint initiatives among stakeholders can further promote the exchange of information and resources, ultimately enhancing the quality and relevance of real-world evidence for Brazilian medtech trials. A case study on information analysis methodology in clinical studies demonstrated how comprehensive information analysis techniques—such as coding, structured memos, and thematic analysis—were employed to understand stakeholder influence on study outcomes.
By utilizing these approaches, researchers efficiently classified stakeholder effects, providing valuable insights for future research. Moreover, the role of patient advocacy groups is paramount; their involvement has been shown to improve study outcomes considerably. Expert opinions affirm that integrating patient perspectives into the research process enriches the data collected and aligns studies more closely with real-world patient experiences.
As one researcher noted, their collaboration with patient partners resulted in an Altmetric score placing their article in the top 5% of all medical articles published, illustrating the visibility and credibility gained through stakeholder engagement.
Additionally, understanding the regulatory functions of INVIMA, the Colombia National Food and Drug Surveillance Institute, is essential. INVIMA oversees the authorization of medical devices and ensures adherence to health regulations, directly impacting the viability and success of research studies in Colombia.
As the landscape of medical research evolves, prioritizing stakeholder involvement will be vital for fostering Medtech innovations that meet the needs of patients and healthcare systems alike. Insights for financiers and organizations on promoting involvement in medical research further emphasize the importance of including diverse stakeholders to enhance the overall efficiency of research studies. Furthermore, the contributions of bioaccess's services to local economies, such as job creation and economic growth, highlight the broader impact of Medtech research on healthcare improvement and international collaboration.
Methodologies for Collecting and Analyzing Real-World Evidence
Gathering and examining real-world evidence for Brazilian medtech trials necessitates a systematic approach to ensure reliability and accuracy—a process in which bioaccess® excels through its extensive clinical study management services in Latin America. With over 20 years of experience in Medtech, bioaccess® employs key methodologies, including observational studies, patient registries, and the analysis of electronic health records. Researchers must establish clear objectives and endpoints for their studies, ensuring that the data collected aligns directly with these goals.
Selecting appropriate statistical methods is crucial, as it accounts for potential biases and confounding factors, thereby enhancing the integrity of the findings. The integration of advanced analytics and machine learning techniques can significantly improve the analysis of extensive datasets, yielding deeper insights into patient outcomes and treatment effectiveness. For instance, hybrid study designs have been successfully utilized in high-profile trials, demonstrating their efficacy in accelerating drug development. By adhering to strict methodologies, researchers can produce high-quality RWE that not only aids regulatory submissions but also guides medical practice, ultimately enhancing healthcare outcomes.
Moreover, the significance of information standards and interoperability is essential for the dependable integration and analysis of real-world evidence across systems, ensuring that the details are both comprehensive and actionable. As pointed out by the US FDA, 'the evidence concerning the usage and potential benefits or risks of a medical product derived from analysis of RWD' is vital for informed decision-making in healthcare environments.
Various regulatory bodies have recognized the importance of RWE, developing guidelines that facilitate its generation and use. These frameworks highlight the necessity for organized information gathering beyond conventional pharmacovigilance, improving study designs and enhancing the overall drug approval process. By utilizing these best practices and adopting a tailored approach, bioaccess® seeks to expedite medical devices more quickly through its expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, allowing Medtech firms to efficiently leverage real-world evidence for Brazilian medtech trials in their research efforts.
Ethical Considerations in Utilizing RWE
Employing Real-World Evidence (RWE) in research necessitates a comprehensive evaluation of ethical issues, particularly regarding patient confidentiality and information security. As we approach 2025, concerns about patient privacy in clinical trials remain significant, with studies indicating that over 70% of participants harbor apprehensions about the use of their information. To address these concerns, researchers must ensure that all information collected is de-identified, adhering to established guidelines that safeguard participant identities while preserving the data's utility for meaningful analysis.
Securing informed consent is paramount; participants must be fully informed about how their information will be utilized, fostering transparency that is essential for maintaining trust with patients and stakeholders. This commitment to openness aligns with bioaccess®'s dedication to information protection, as outlined in our grievance procedures, ensuring client concerns are addressed with compliance and transparency. As Elliot E. Maxwell, J.D., noted, "This paper will attempt to put the use of healthcare information into the larger context of transforming health care by increasing openness."
Moreover, researchers should strictly adhere to ethical guidelines established by regulatory bodies, designed to minimize risks to participants. A case study on the challenges of data de-identification illustrates the complexities involved in ensuring that high-dimensional datasets remain useful for research while mitigating re-identification risks. By adopting a standardized approach to de-identification that complies with HIPAA guidelines, researchers can enhance the credibility of their RWE studies.
This is particularly relevant given bioaccess®'s over 15 years of experience in the Medtech sector, underscoring the importance of ethical practices in research. Prioritizing these ethical considerations not only fortifies the integrity of research but also cultivates a culture of responsibility within the research community. As the medical technology landscape evolves, the importance of patient privacy in research involving real-world evidence for Brazilian medtech trials cannot be overstated, ensuring that ethical practices remain at the forefront of research initiatives. Furthermore, a nationwide educational initiative is essential to underscore the benefits of privacy-compliant health research, further emphasizing the significance of ethical considerations in clinical studies.
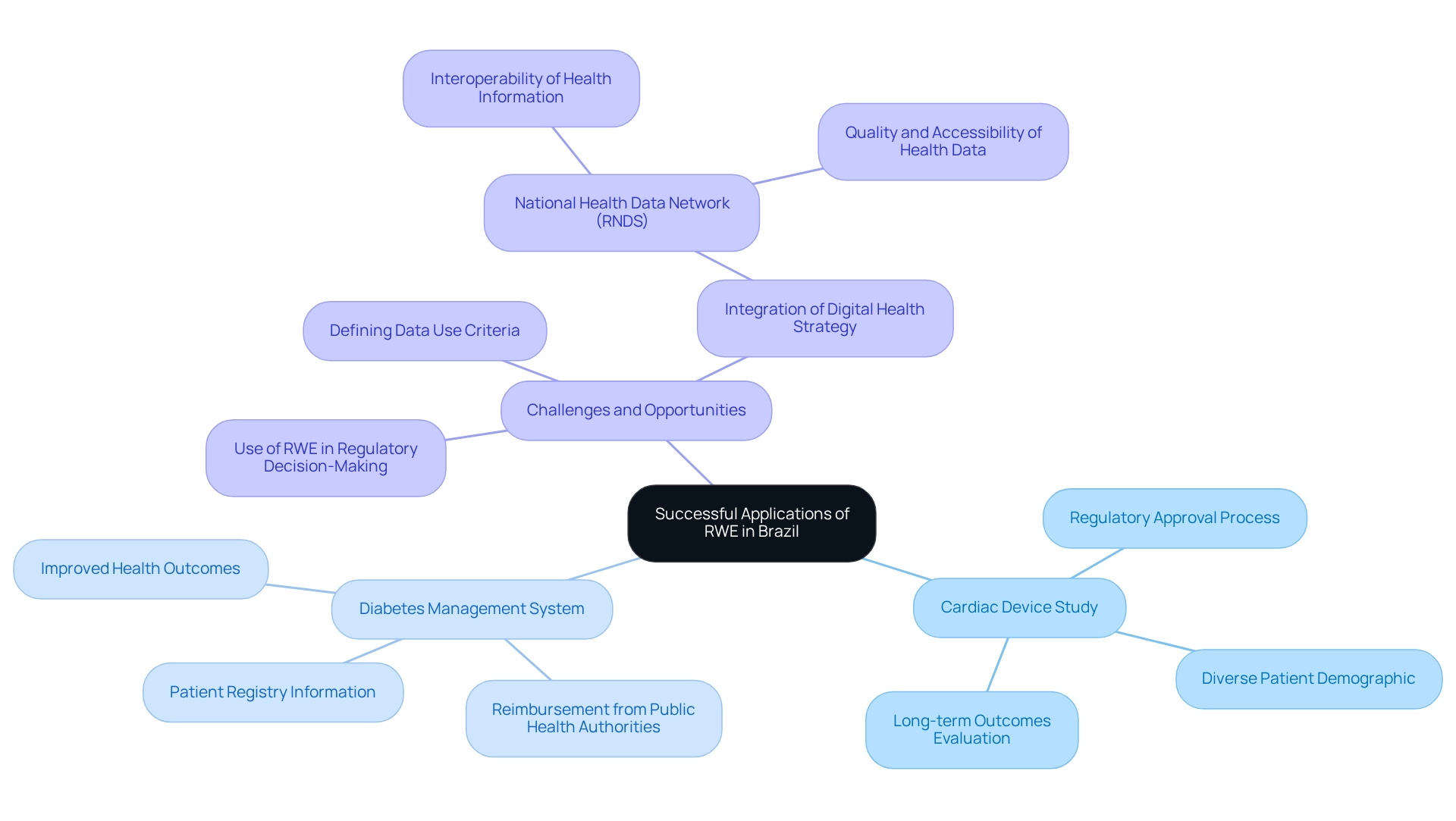
Case Studies: Successful Applications of RWE in Brazil
Numerous case studies illustrate the effective use of real-world evidence for Brazilian medtech trials, particularly those managed by bioaccess®, which boasts over 20 years of experience in the Medtech field. A notable example is a recent investigation involving a cardiac device that employed RWE to evaluate long-term outcomes across a diverse patient demographic. This approach not only provided robust information but also expedited the regulatory approval process, showcasing the potential of RWE to streamline pathways to market.
Another significant case involved a diabetes management system that utilized patient registry information to demonstrate improved health outcomes. This evidence was crucial in securing reimbursement from public health authorities, underscoring the role of RWE in facilitating financial support for innovative solutions.
However, it is important to note that RWE is primarily utilized for pharmacovigilance and academic research in South America, with less frequent application in health technology assessment decision-making or pricing negotiations. Challenges persist in establishing criteria for information use to ensure that the generated information is adequate for guiding regulatory decision-making by Anvisa.
As Jeffrey Bradley, MD, noted, "Our special thanks to Jeffrey Bradley, MD (Department of Radiation Oncology, Emory University School of Medicine at Atlanta) for valuable discussion and insights," emphasizing the importance of expert perspectives in understanding RWE's role in regulatory processes.
Additionally, the Digital Health Strategy and the National Health Data Network (RNDS) aim to integrate and enhance the interoperability of health information across public and private health institutions in Brazil. The RNDS is expected to become a national reference for health data collection, improving the quality and accessibility of health information for regulatory purposes.
These examples highlight the essential requirement to incorporate RWE into study designs. By doing so, researchers can present compelling evidence of a device's effectiveness, foster collaboration with key stakeholders, and bolster the overall credibility of their research efforts. As the landscape of Medtech continues to evolve, leveraging RWE will be essential for navigating regulatory frameworks and achieving successful outcomes.
With bioaccess® leading the charge in clinical research, their expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, along with their customized approach and commitment to innovation and regulatory excellence, positions them as a key partner in advancing Medtech innovation in Latin America.
Best Practices and Recommendations for RWE in Brazilian Medtech Trials
To effectively harness Real-World Evidence (RWE) in Brazilian Medtech trials, stakeholders should adopt the following best practices:
-
Engage Stakeholders Early: Involving key stakeholders—such as patients, regulatory agencies, and healthcare providers—at the outset of the study design process is crucial. This approach guarantees alignment with patient requirements and regulatory expectations, fostering a cooperative atmosphere that enhances study relevance.
-
Employ Rigorous Methodologies: Robust methodologies for information collection and analysis are essential to bolster the reliability of RWE. Real-World Data (RWD) is defined as information gathered outside the setting of randomized controlled studies, produced during standard medical practice. This data is vital for generating insights that reflect real-world usage and outcomes. Leveraging bioaccess's expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) can significantly enhance the rigor of these methodologies.
-
Prioritize Ethical Considerations: Safeguarding patient privacy and ensuring informed consent throughout the research process is paramount. Ethical considerations are crucial in upholding trust and integrity in clinical studies, particularly when handling sensitive health information.
-
Utilize Effective Case Studies: Referencing successful case studies that illustrate the effective application of RWE in previous studies can provide valuable insights. For example, the case study titled 'Validation of Tools and Processes for Real-World Evidence for Brazilian medtech trials' highlights the critical need for validating the tools and processes used in generating real-world evidence. Such examples can inform trial designs and demonstrate the value of RWE to regulators and payers, ultimately facilitating smoother approval processes.
-
Stay Informed on Regulatory Guidelines: Keeping abreast of evolving regulatory guidelines and best practices related to RWE is essential. As noted by the US Food and Drug Administration, evidence regarding the usage and potential advantages or dangers of a medical product can be obtained through analysis of RWD. This knowledge is crucial for ensuring adherence and optimizing the application of RWE in medical research, especially as the regulatory environment continues to evolve. Understanding the role of INVIMA as a Level 4 health authority in Colombia can also provide valuable insights into regulatory expectations.
By applying these suggestions, Medtech firms can significantly enhance the standard and influence of their studies in Brazil, generating real-world evidence that ultimately leads to more efficient medical devices meeting the needs of patients and healthcare systems. The growing demand for validation of tools and processes used to generate RWE underscores the importance of these practices in the current regulatory environment. With over 20 years of experience in the Medtech field, bioaccess® offers a customized approach to navigating clinical trials, ensuring that all aspects of the study are managed effectively.
Conclusion
The integration of Real-World Evidence (RWE) into Medtech trials signifies a transformative shift that enhances regulatory decision-making and patient care. As emphasized throughout this article, RWE is not merely a supplementary tool; it is a fundamental component in demonstrating the efficacy and safety of medical devices within real-world contexts. By harnessing insights from Real-World Data (RWD), stakeholders can streamline the development process, enabling informed decisions that ultimately expedite patient access to innovative healthcare solutions.
Nonetheless, the successful implementation of RWE in Brazil faces several challenges. Navigating the regulatory landscape, addressing logistical obstacles, and ensuring ethical compliance necessitate a strategic approach. Engaging stakeholders early in the trial design process emerges as a best practice that not only enriches the research but also aligns it more closely with patient needs and regulatory expectations. The experiences highlighted in various case studies underscore the critical importance of collaboration and the tailored application of RWE methodologies across diverse healthcare environments.
In summary, the future of Medtech trials in Brazil relies on the effective utilization of RWE, underpinned by robust methodologies and ethical considerations. By adopting best practices and learning from successful implementations, Medtech companies can enhance the credibility and relevance of their research. As the industry evolves, a steadfast commitment to integrating RWE will be vital in addressing the demands of regulators and patients alike, ultimately leading to improved healthcare outcomes and innovations that resonate with the needs of the population.
Frequently Asked Questions
What is Real-World Evidence (RWE)?
Real-World Evidence (RWE) is defined as clinical evidence obtained from the analysis of Real-World Data (RWD), which includes information gathered outside conventional clinical studies.
How is RWE used in Brazilian medtech trials?
RWE is increasingly used in Brazilian medtech trials to inform regulatory decisions, improve patient outcomes, and support the development of medical devices by providing insights into device performance in real-world settings.
Why is RWE important in medical device regulation?
RWE is crucial in medical device regulation as it helps regulatory bodies, including the FDA, make informed decisions. There was a 193% increase in RWE use for regulatory decisions from 2015 to 2017, highlighting its growing significance.
How do pharmaceutical companies benefit from RWE?
Pharmaceutical companies utilize RWE to enhance patient and provider experiences, particularly by understanding side effects and improving educational outreach, which fosters better brand equity and patient trust.
What role does bioaccess® play in Brazilian medtech trials?
bioaccess® integrates RWE into trial management by offering comprehensive services such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, positioning itself as a leader in Medtech research in Brazil.
What challenges do Medtech studies face in Brazil?
Medtech studies in Brazil face challenges such as intricate regulatory requirements, prolonged approval timelines, logistical difficulties, and cultural factors that affect patient recruitment, all of which can complicate the execution of studies.
What is the typical approval duration for clinical studies in Brazil?
The typical approval duration for clinical studies in Brazil can extend to 12 months or more, complicating the process for Medtech firms.
How does cultural understanding impact patient recruitment in Brazil?
Effective patient recruitment strategies require a nuanced understanding of local populations to ensure adequate participation rates in clinical studies.
What services does bioaccess® provide to navigate Medtech challenges?
bioaccess® provides services such as feasibility assessments, site selection, compliance evaluations, trial setup, import permits, nationalization of investigational devices, project management, and reporting on adverse events.
What is the broader impact of Medtech clinical studies on local economies in Brazil?
Medtech clinical studies positively impact local economies through job creation and healthcare improvements, emphasizing the importance of international collaboration in promoting global health advancement.




