Overview
This article delves into best practices in Medtech trial design, underscoring the critical nature of effective study design, regulatory compliance, and the integration of technology to optimize trial outcomes. It articulates essential components such as:
- Precise research questions
- Suitable methodologies
- Patient-centric endpoints
Furthermore, it emphasizes the significance of collaboration among stakeholders and the deployment of advanced technologies, including:
- AI
- Electronic data capture
to enhance efficiency and uphold data integrity in clinical trials.
Introduction
In the rapidly evolving landscape of medical technology, the design of clinical trials is paramount in determining the success of new innovations. With regulatory compliance and patient safety at the forefront, Medtech companies must navigate a myriad of complexities to ensure that their trials yield meaningful results.
From defining precise research questions to optimizing endpoints and leveraging cutting-edge technology, each component of trial design is pivotal in generating reliable data. As the industry shifts towards a more patient-centric approach, understanding the fundamentals of clinical trial design becomes essential for stakeholders aiming to enhance efficiency and achieve impactful outcomes.
This article delves into the critical elements of clinical trial design in Medtech, exploring best practices, emerging trends, and the transformative role of collaboration and technology in shaping the future of healthcare solutions.
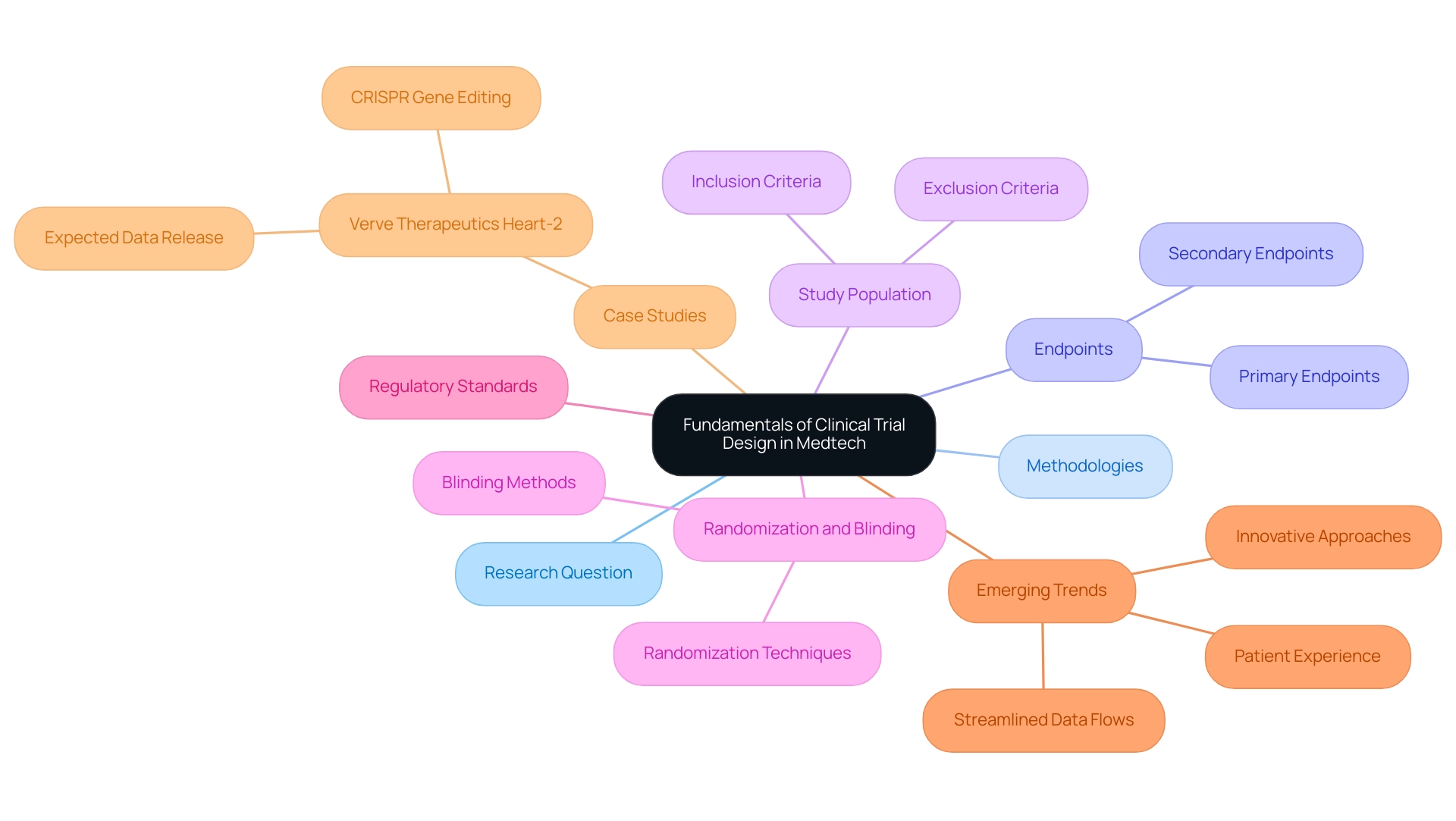
Fundamentals of Clinical Trial Design in Medtech
Effective clinical study design in the Medtech sector is pivotal for advancing medical technologies and aligning with best practices in trial design. A successful experiment relies on several essential components, including the formulation of a precise research question, the selection of appropriate methodologies, and the establishment of clear endpoints. A well-structured protocol serves as the foundation, detailing the study's objectives, design, and statistical analysis plan.
Key considerations include:
- Study Population: Defining inclusion and exclusion criteria meticulously is crucial to target the appropriate patient population, thereby enhancing the relevance and applicability of the trial results.
- Randomization and Blinding: Implementing randomization minimizes bias, enhancing the validity of results. Additionally, blinding further mitigates bias in treatment allocation, ensuring that outcomes are assessed objectively.
- Endpoints: Establishing primary and secondary endpoints that are clinically relevant and measurable is essential. These endpoints should align closely with the study objectives to provide meaningful insights into the product's efficacy and safety.
Following these essential principles guarantees adherence to regulatory standards and promotes the creation of significant information that aids product development and market entry. Recent trends in research design emphasize the importance of patient experience and streamlined information flows, significantly enhancing the efficiency and effectiveness of studies. Innovative approaches are being adopted to simplify site experiences and reduce manual activities, ultimately improving patient care.
Danish Mairaj, principal engineer of medical device design at RESYCA, noted, "This is one of the areas where AI can utilize a lot of information, but gaining insights from this information is only possible if you have certain use cases being implemented in relevant facilities." This emphasizes the essential function of AI in extracting practical insights from research data.
In this context, collaborating with bioaccess® can significantly improve your research endeavors. With over 20 years of experience in medical technology, bioaccess® specializes in managing a range of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Their comprehensive clinical study management services include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
A notable example is Verve Therapeutics, which is pursuing a CRISPR gene editing approach to prevent heart attacks with a single infusion targeting the PCSK9 gene. Their aim is to provide a single drug infusion to prevent heart attacks for life. Initial data from their ongoing study is expected in the first half of 2025, which could affect investor sentiment and guide future strategies in the medical technology landscape.
By incorporating these best practices and remaining aware of emerging trends, medical technology firms can create studies that not only fulfill regulatory standards but also aid in the progression of healthcare solutions.
Navigating Regulatory Compliance in Medtech Trials
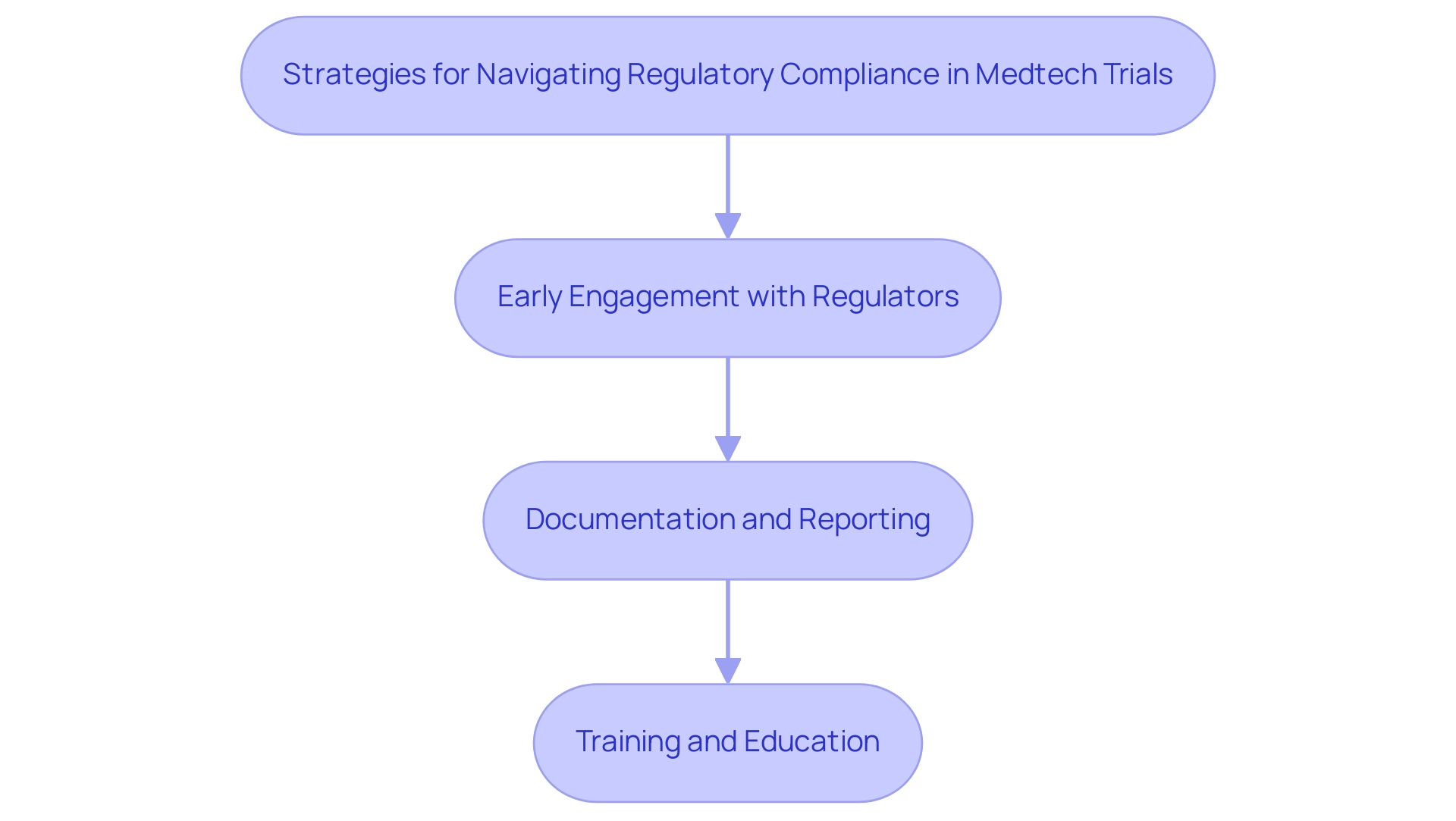
Navigating regulatory compliance in medical technology studies requires a comprehensive understanding of the guidelines established by authorities such as the FDA and EMA. As the landscape evolves, particularly with the anticipated regulations for 2025, Medtech companies must adopt key strategies to ensure compliance and facilitate successful trial outcomes:
- Early Engagement with Regulators: Initiating discussions with regulatory bodies at the outset of the trial design process is crucial. This proactive approach allows companies to clarify expectations and requirements, ultimately streamlining the approval process.
- Documentation and Reporting: Keeping detailed records of all study activities—including protocol amendments, adverse events, and information management practices—is essential for ensuring transparency and accountability. This thorough documentation not only supports compliance but also enhances the integrity of the research data.
- Training and Education: Providing ongoing training for research staff on regulatory requirements and Good Clinical Practice (GCP) fosters a culture of compliance within the organization. Regular educational updates ensure that all team members are well-versed in the latest regulations and best practices.
Implementing these strategies can significantly mitigate the risks associated with best practices in Medtech trial design. As highlighted by Celeste Maksim, "The EU MDR’s date of application has passed, but activities such as PMCF require a nonstop effort to meet the new regulation’s requirements." Furthermore, Gartner predicts that by 2025, a third of all nations will have laws specifically concerning ransomware payments, underscoring the broader regulatory challenges that medical technology firms may encounter.
For instance, South Korea's Digital Medical Products Act, effective January 2025, establishes classification standards for digital medical technologies, emphasizing the need for rigorous oversight to ensure safety and efficacy. By aligning with such evolving regulations, Medtech companies can enhance their likelihood of successful testing outcomes and ultimately advance their medical devices to market more efficiently. Bioaccess® provides an extensive range of management services for research projects, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, to assist these efforts and aid companies in navigating the intricacies of regulatory adherence effectively.
To learn more about how Bioaccess can assist you, BOOK A MEETING today.
Optimizing Trial Endpoints for Effective Outcomes
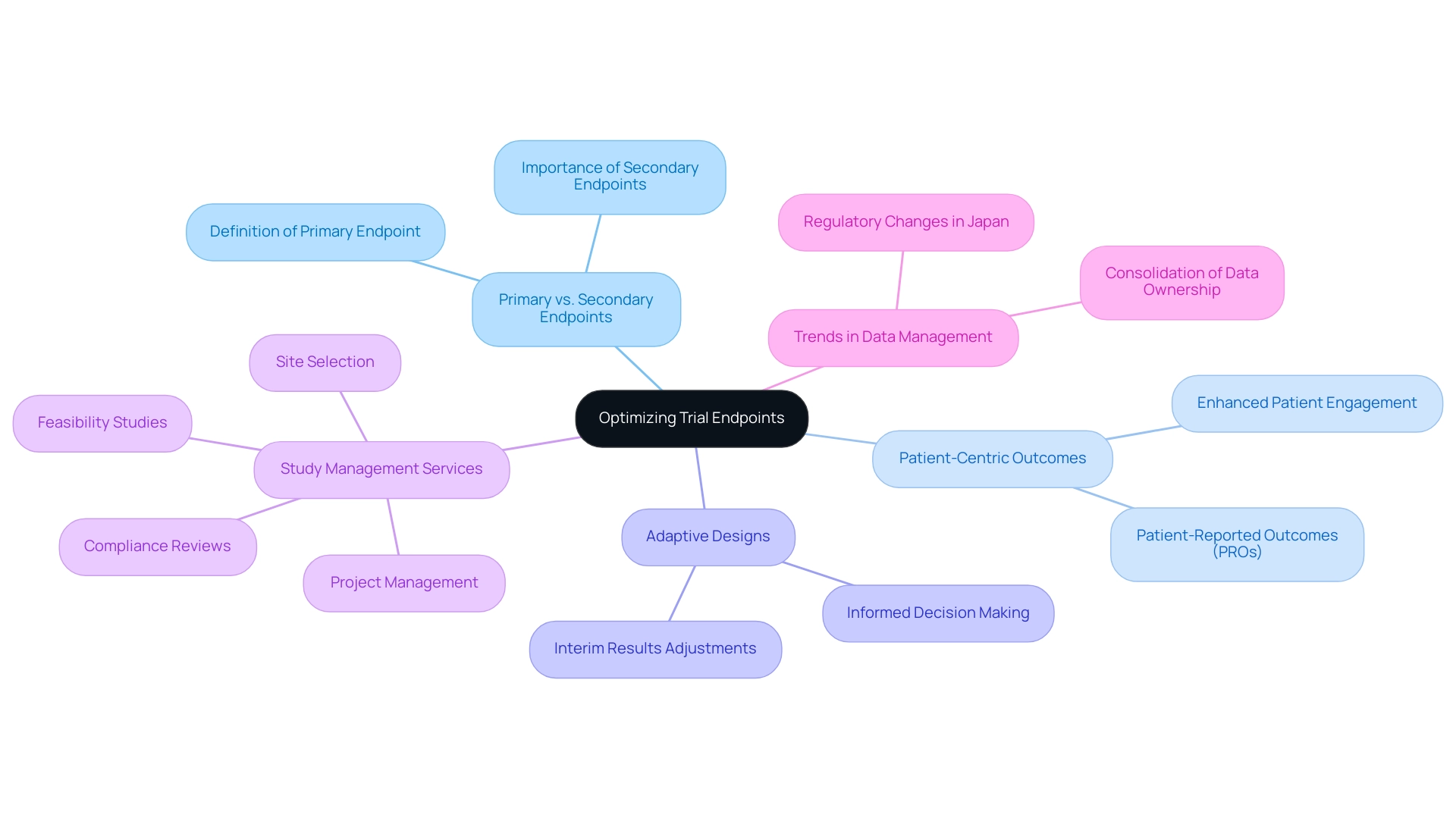
Choosing and refining study endpoints is a vital component of research design, reflecting best practices in medtech trial design by necessitating a balance between medical significance and statistical strength. Key practices in this area include:
- Primary vs. Secondary Endpoints: Clearly differentiating between primary and secondary endpoints is essential. The primary endpoint should represent the most crucial measure of the study's success, guiding the overall evaluation of the treatment's efficacy.
- Patient-Centric Outcomes: Incorporating patient-reported outcomes (PROs) is vital for capturing the patient's perspective on treatment effectiveness and quality of life. As noted by Peng Lu, chief medical officer of Dutch biotech Pharvaris, "Some diseases, like hereditary angioedema (HAE), rely on patient-reported outcomes (PROs) to evaluate the effectiveness of novel therapeutics in research studies." In 2025, studies indicate that experiments emphasizing PROs have shown enhanced patient engagement and satisfaction, leading to more meaningful results.
- Adaptive Designs: Utilizing adaptive study designs can significantly enhance the efficiency and relevance of research. These designs permit adjustments based on interim results, enabling researchers to make informed choices that can enhance outcomes.
Alongside these best practices, extensive research study management services, such as those offered by bioaccess, play an essential role in guaranteeing successful execution. These services include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, encompassing obtaining ethics committee approvals and nationalization of investigational devices. By efficiently overseeing these elements, Medtech firms can navigate the intricacies of research studies, ensuring compliance with regulatory standards while optimizing the potential for significant outcomes.
Recent trends indicate a shift towards sponsors consolidating information management and owning their information, rather than relying solely on contract research organizations (CROs). This shift is motivated by the demand for enhanced transparency and oversight over medical information, which ultimately results in better study quality and patient outcomes. A case study titled "Navigating Industry Changes and Information Management Challenges" highlights how sponsors who embraced this model effectively addressed information management challenges, resulting in more resilient and strategic approaches to clinical research.
Furthermore, the Japanese government's recent actions permitting Japanese locations to be part of global multi-center Phase III studies without earlier Phase I Japanese patient information demonstrate the evolving study designs and regulatory landscapes that medical technology firms must manage.
By concentrating on best practices in medtech trial design and utilizing extensive study management services, such as those provided by bioaccess, medical technology companies can develop studies that not only adhere to regulatory standards but also generate valuable insights into the product's performance. This approach ensures that the studies are both scientifically rigorous and patient-centered, ultimately contributing to job creation, economic growth, and healthcare enhancement in local economies.
Leveraging Technology for Enhanced Trial Design
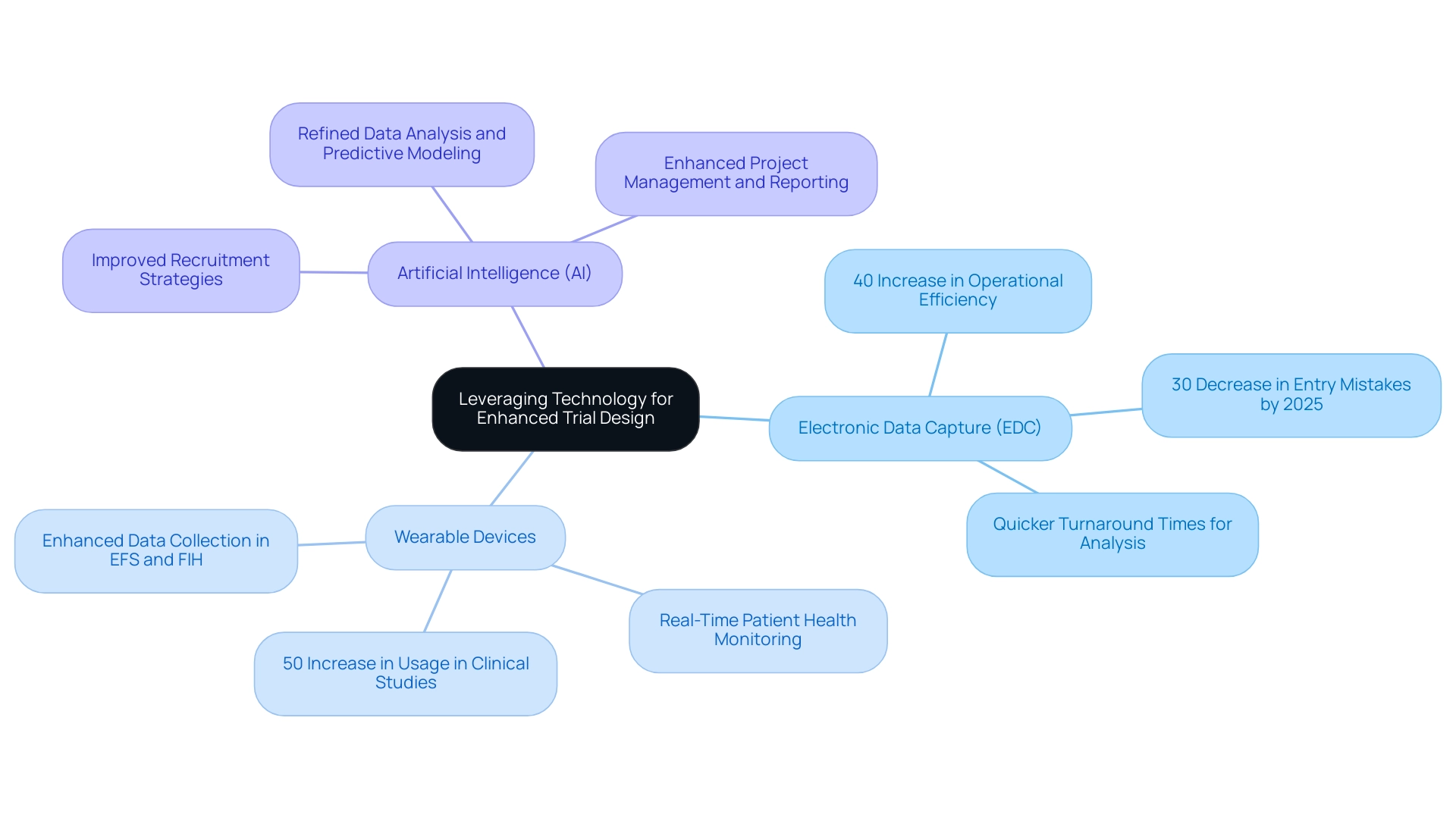
Utilizing technology in clinical study design is essential for enhancing efficiency and ensuring information integrity, particularly when adhering to best practices in medtech trial design within the comprehensive clinical study management services offered by bioaccess®. Key technologies that Medtech companies should consider include:
- Electronic Data Capture (EDC): Implementing EDC systems can significantly streamline data collection processes, minimizing the errors commonly associated with traditional paper-based methods. By 2025, studies suggest that experiments employing EDC will experience a 30% decrease in entry mistakes, resulting in more dependable outcomes. Bioaccess® has successfully incorporated EDC into its study management, leading to a remarkable 40% increase in operational efficiency for studies.
- Wearable Devices: The integration of wearable technology allows for real-time observation of patient health metrics, providing essential information that can enhance study results. Recent statistics indicate that the use of wearables in clinical studies has surged by 50% in the past year, reflecting their growing significance in gathering continuous patient information. Bioaccess® employs these devices to ensure comprehensive data collection during Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
- Artificial Intelligence (AI): Utilizing AI algorithms for data analysis and predictive modeling can refine study design and improve participant recruitment strategies. As the research study environment becomes increasingly competitive, leveraging AI can assist in identifying suitable candidates more effectively, thus accelerating the process. Bioaccess® employs AI to enhance its project management and reporting capabilities, ensuring that studies are not only compliant but also strategically positioned for success.
The profound impact of these technologies is evident in the best practices of medtech trial design. For instance, a recent case study titled "Focus on Commercial Outcomes in Drug Development" highlighted the biopharma industry's shift towards emphasizing commercial outcomes, underscoring the necessity for studies to not only meet regulatory endpoints but also ensure commercial viability. This shift emphasizes the importance of best practices in medtech trial design to optimize development journeys through advanced technologies, as Max Baumann noted, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
Moreover, the advantages of EDC in medical technology studies are particularly noteworthy. By 2025, experiments that adopted EDC reported a 40% increase in operational efficiency, demonstrating how technology can facilitate smoother workflows and enhance information quality. Successful applications of EDC by bioaccess® have shown that medical technology firms can achieve quicker turnaround times for information analysis, ultimately resulting in swifter decision-making and improved study outcomes.
Furthermore, as the healthcare market becomes increasingly crowded, it is imperative for Medtech companies to recognize the fundamental business model challenges highlighted by Max Baumann and to implement best practices in medtech trial design. By embracing these technological advancements and exploring emerging strategies such as data contracts for contemporary data sharing, bioaccess® can significantly elevate the overall quality and efficiency of research studies, positioning itself for success in a competitive market while contributing to local economies through job creation and healthcare improvement.
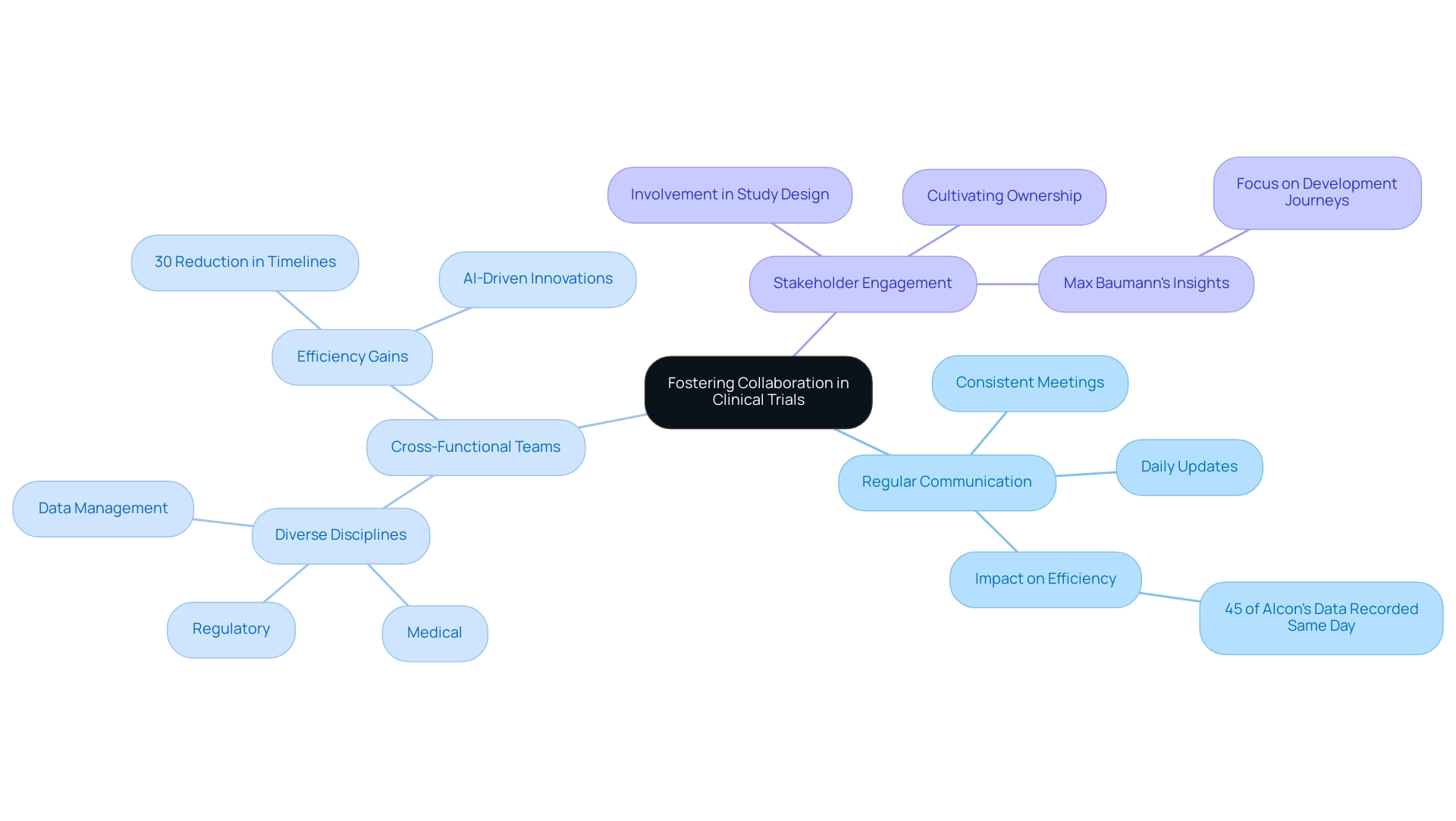
Fostering Collaboration Among Stakeholders in Clinical Trials
Promoting cooperation among stakeholders in clinical studies is essential for enhancing efficiency and achieving successful outcomes. Key strategies include:
- Regular Communication: Establishing consistent meetings and updates is vital for keeping all stakeholders informed and aligned on trial progress and challenges. This strategy not only fosters transparency but also builds trust among team members. For instance, statistics indicate that 45% of Alcon's information is recorded on the same day as the visit date, underscoring the impact of prompt communication on efficiency. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, highlighted the significance of communication during his experience with bioaccess® in their first human study in Colombia, emphasizing that effective dialogue can lead to improved outcomes.
- Cross-Functional Teams: Forming cross-functional teams that encompass various disciplines—such as medical, regulatory, and data management—can greatly enhance collaboration and problem-solving capabilities. Research shows that organizations utilizing such teams experience a notable increase in efficiency during experiments, with data indicating that effective collaboration can result in a 30% reduction in timelines. Moreover, the case study titled 'AI-Driven Innovations in Drug Development' illustrates how AI and machine learning are becoming integral to clinical study design, further enhancing collaboration and efficiency.
- Stakeholder Engagement: Actively involving stakeholders—including patients, investigators, and regulatory bodies—in the study design process ensures that their perspectives and needs are considered. This engagement is crucial, as it cultivates a sense of ownership and commitment among all parties involved. As Max Baumann, Head of Execution, states, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success," highlighting the importance of collaboration and stakeholder engagement. The partnership with LATAM CRO specialists, as demonstrated in bioaccess®'s methodology, exemplifies how local expertise can enhance study design and execution.
By implementing these strategies, healthcare technology firms can cultivate a collaborative environment that adheres to best practices in medtech trial design, thereby boosting efficiency in experiments and enhancing overall effectiveness. This ultimately leads to improved patient outcomes and successful product commercialization. Furthermore, with upcoming events related to infectious disease research trends, the necessity of cooperation in current research contexts is more critical than ever, contributing to job creation and economic development in local communities. Bioaccess® specializes in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, backed by over 20 years of expertise in medical technology, ensuring a comprehensive approach to clinical trials that positively impacts local economies.
The Role of Post-Market Studies in Medtech Trial Design
Post-market studies are essential in the life cycle of medical devices, offering crucial insights into their long-term safety and effectiveness. As the Medtech landscape evolves, particularly in 2025, several key considerations emerge:
-
Study Design: It is imperative to design post-market studies that specifically address questions regarding the device's performance in real-world settings. This includes evaluating long-term outcomes and identifying any adverse events that may arise over time. As Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, leveraging technology can significantly enhance study designs. He stated, "We did a small AI initiative to see if we can generate meaningful test information for setting up and validating our systems." It turns out we can. An algorithm that we created examines previous studies we established, learns from the actual information gathered, and utilizes it to produce something we can apply for new studies.
-
Information Gathering: Leveraging registries and electronic health records is vital for efficient and comprehensive information gathering. This approach ensures a robust analysis of post-market performance, allowing for a clearer understanding of how devices function in diverse patient populations. The integration of technologies like generative AI is expected to enhance operational efficiencies and drive innovations in the life sciences industry, making data collection more effective.
-
Regulatory Compliance: Adhering to regulatory requirements is crucial, as post-market studies are often mandated by authorities to continuously monitor device safety and effectiveness after approval. In 2025, with 77% of US respondents anticipating increased regulatory emphasis on sustainability, compliance will also encompass environmental considerations, further complicating the landscape. Additionally, 83% of non-US survey participants believe the European Union’s Corporate Sustainability Reporting Directive will significantly affect their 2025 strategies, highlighting the need for healthcare technology firms to stay informed about evolving regulations.
By prioritizing post-market studies, medical technology firms can significantly enhance their understanding of device performance, ensuring ongoing compliance with regulatory standards while contributing to the overall safety and efficacy of medical technologies. This proactive approach not only supports regulatory obligations but also fosters trust among stakeholders and patients alike. Furthermore, as illustrated by TATEEDA Global's emphasis on monitoring healthcare IT market trends, implementing best practices in medtech trial design and aligning study designs with industry demands is essential for success in the evolving medical technology landscape.
At bioaccess®, with over 20 years of experience in Medtech, we specialize in comprehensive research management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies. Our customized approach ensures that your clinical trials are conducted with the highest level of expertise and compliance. Katherine Ruiz, our expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, is dedicated to guiding your studies through the complexities of regulatory landscapes, particularly in the Latin American market.
Conclusion
In the dynamic realm of Medtech, the design of clinical trials stands as a crucial determinant of the success of new medical innovations. This article underscores the essential components of effective trial design, encompassing the formulation of precise research questions, the selection of appropriate methodologies, and the establishment of clear endpoints that align with both regulatory standards and patient needs. The significance of incorporating patient-centric outcomes and adaptive designs is highlighted, reflecting the industry's shift towards a more holistic approach in clinical research.
Moreover, leveraging technology, such as electronic data capture and AI, emerges as a vital means to enhance trial efficiency and data integrity. As Medtech companies navigate the complexities of regulatory compliance, proactive engagement with regulatory bodies and ongoing staff training are indispensable strategies that mitigate risks and facilitate successful outcomes.
Ultimately, fostering collaboration among stakeholders is identified as a key strategy for improving trial efficiency and effectiveness. By embracing these best practices and emerging trends, Medtech companies can not only ensure compliance with regulatory requirements but also contribute to the advancement of healthcare solutions. As the industry continues to evolve, the integration of innovative approaches and technologies will be instrumental in shaping the future of clinical trials, paving the way for groundbreaking medical advancements that not only meet regulatory standards but also significantly enhance patient care and outcomes.
Frequently Asked Questions
Why is effective clinical study design important in the Medtech sector?
Effective clinical study design is crucial for advancing medical technologies and ensuring alignment with best practices in trial design. It helps in formulating precise research questions, selecting appropriate methodologies, and establishing clear endpoints.
What are the essential components of a successful clinical study?
A successful clinical study relies on several components, including a well-structured protocol that details the study's objectives, design, and statistical analysis plan, as well as the formulation of a precise research question, selection of methodologies, and establishment of clear endpoints.
How should the study population be defined in clinical studies?
The study population should be defined by meticulously outlining inclusion and exclusion criteria to target the appropriate patient population, enhancing the relevance and applicability of the trial results.
What is the role of randomization and blinding in clinical studies?
Randomization minimizes bias and enhances the validity of results, while blinding further mitigates bias in treatment allocation, ensuring that outcomes are assessed objectively.
What are endpoints in clinical studies, and why are they important?
Endpoints are primary and secondary outcomes that are clinically relevant and measurable. They should align closely with the study objectives to provide meaningful insights into the product's efficacy and safety.
How do recent trends in research design impact clinical studies?
Recent trends emphasize the importance of patient experience and streamlined information flows, which enhance the efficiency and effectiveness of studies. Innovative approaches are being adopted to simplify site experiences and reduce manual activities.
How does AI contribute to clinical study design?
AI plays an essential role in extracting practical insights from research data, which can help in gaining valuable information for study design and implementation.
What services does bioaccess® offer for clinical study management?
Bioaccess® specializes in managing a range of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies. Their services include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
Can you provide an example of a current clinical study in the Medtech sector?
Verve Therapeutics is pursuing a CRISPR gene editing approach to prevent heart attacks with a single infusion targeting the PCSK9 gene. Initial data from their ongoing study is expected in the first half of 2025.
What strategies can Medtech companies adopt to navigate regulatory compliance?
Key strategies include early engagement with regulators, thorough documentation and reporting of study activities, and providing ongoing training for research staff on regulatory requirements and Good Clinical Practice (GCP).
Why is documentation important in clinical studies?
Keeping detailed records of all study activities enhances transparency and accountability, supports compliance, and improves the integrity of the research data.
What challenges do Medtech companies face regarding regulations?
Medtech companies face evolving regulatory challenges, such as compliance with the EU MDR and new regulations like South Korea's Digital Medical Products Act, which emphasize rigorous oversight to ensure safety and efficacy.
How can Bioaccess® assist with regulatory compliance in clinical studies?
Bioaccess® provides a range of management services for research projects, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, helping companies navigate regulatory adherence effectively.

