Overview
Building successful Medtech partnerships in Ecuador necessitates a comprehensive understanding of the local market, regulatory environment, and cultural dynamics. This article underscores the critical need for Medtech companies to:
- Conduct thorough research on market conditions
- Engage with local stakeholders
- Adhere to regulatory requirements
By doing so, these companies can strategically position themselves to leverage Ecuador's unique advantages for clinical trials and effectively enhance healthcare solutions.
Introduction
Ecuador's Medtech landscape is rapidly evolving, presenting both challenges and opportunities for companies aiming to establish a presence in this promising market. With a projected surge in demand for medical technologies—particularly in nephrology and urology—understanding the intricacies of this industry is essential.
Companies must navigate the regulatory environment set forth by the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA) while identifying local healthcare needs and trends. A multifaceted approach is crucial for success.
Moreover, the strategic advantages of conducting clinical trials in Ecuador, such as cost-effectiveness and a diverse patient population, position the country as an attractive hub for research and innovation. Establishing partnerships with local institutions and staying informed about regulatory changes will be key to thriving in this dynamic landscape.
As the Medtech industry in Ecuador continues to grow, those who are well-prepared will undoubtedly lead the way in transforming healthcare solutions for the local population.
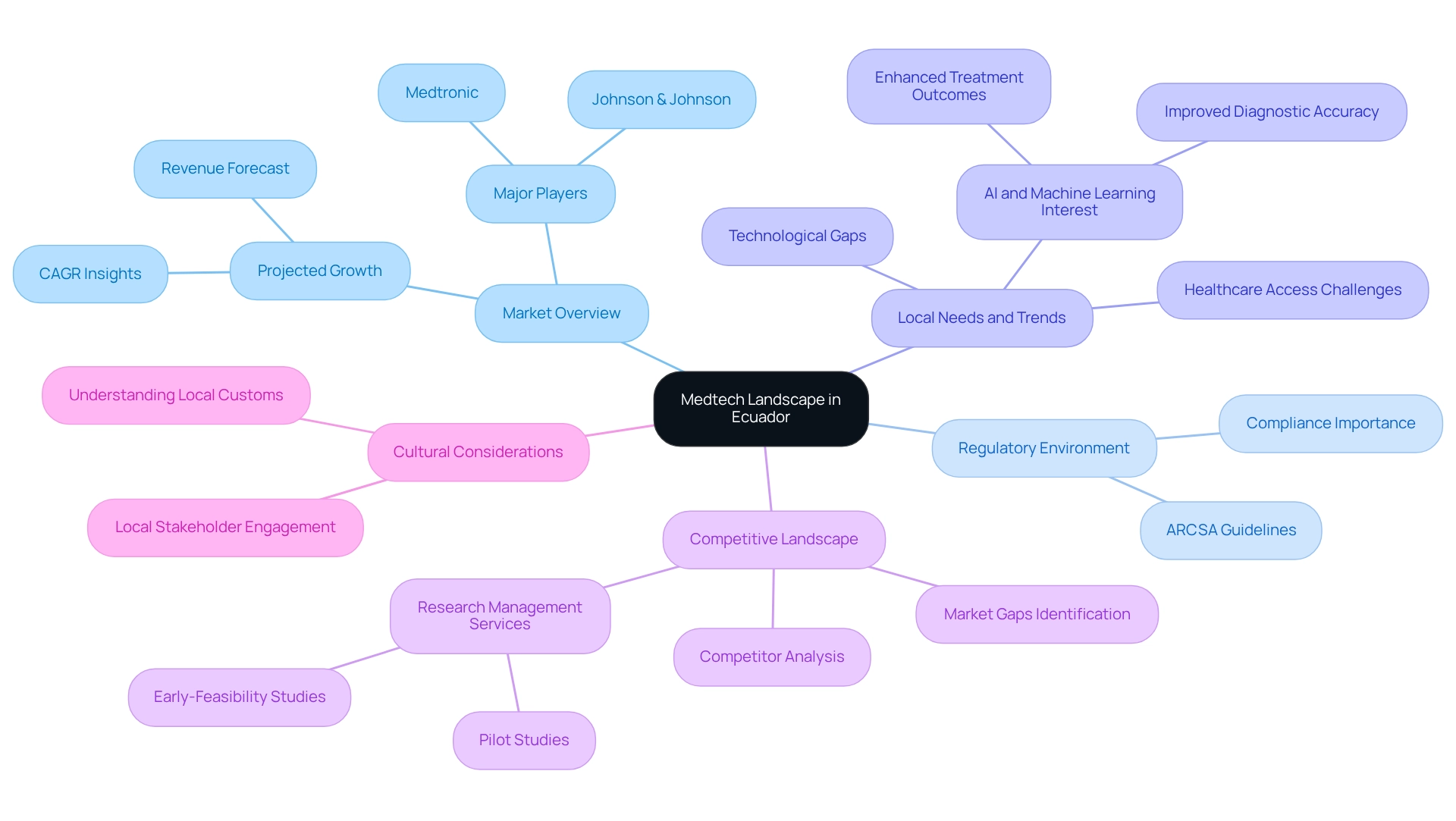
Understand the Medtech Landscape in Ecuador
To effectively navigate the Medtech landscape in Ecuador, it is essential to research several key areas:
- Market Overview: Acquire a thorough insight into the present condition of the medical technology sector in the country. The market is projected to experience significant growth, with revenues from nephrology and urology devices expected to rise steadily from 2021 to 2031. Specifically, the market is forecasted to reach $X million by 2031, reflecting a compound annual growth rate (CAGR) of Y%. Familiarizing yourself with market size, growth rates, and major players is crucial for building medtech partnerships in Ecuador, providing a solid foundation for strategic planning.
- Regulatory Environment: Familiarize yourself with the regulations governing medical devices and clinical trials in the country. The Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA) serves as the primary regulatory body, and adherence to their guidelines is crucial for ensuring compliance and successful market entry. As Yolanda Mega, Operations Manager, states, "Get in touch with us. We are happy to help," emphasizing the importance of understanding regulatory requirements.
- Local Needs and Trends: Identify the specific healthcare needs of the Ecuadorian population. By building medtech partnerships in Ecuador, understanding prevalent diseases, healthcare access challenges, and technological gaps will enable you to tailor your Medtech solutions effectively. The rising interest in artificial intelligence and machine learning in healthcare highlights a growing demand for innovative technologies that can enhance diagnostic accuracy and treatment outcomes. For instance, a recent case study indicates that the integration of AI technologies is expected to significantly improve healthcare solutions and patient care in Ecuador.
- Competitive Landscape: Conduct a thorough analysis of existing competitors and their offerings. Notably, major global players like Medtronic and Johnson & Johnson are among the top three largest medical technology companies globally, which underscores the competitive nature of the market. This assessment will help you pinpoint market gaps and identify opportunities for differentiation, which is essential for building medtech partnerships in Ecuador and allowing your organization to position itself strategically within the sector. Furthermore, utilizing extensive research study management services, like those provided by bioaccess®, can improve your competitive advantage. Their expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies ensures that your clinical trials are managed effectively, contributing to successful market entry and compliance while also fostering job creation and healthcare improvement in the local economy.
- Cultural Considerations: Recognize the cultural elements that might affect healthcare provision and the acceptance of new technologies in that country. Engaging with local stakeholders can provide valuable insights into these dynamics, facilitating smoother integration of your Medtech solutions into the healthcare ecosystem. Understanding local customs and practices will be essential in ensuring that your innovations are well-received and effectively utilized.
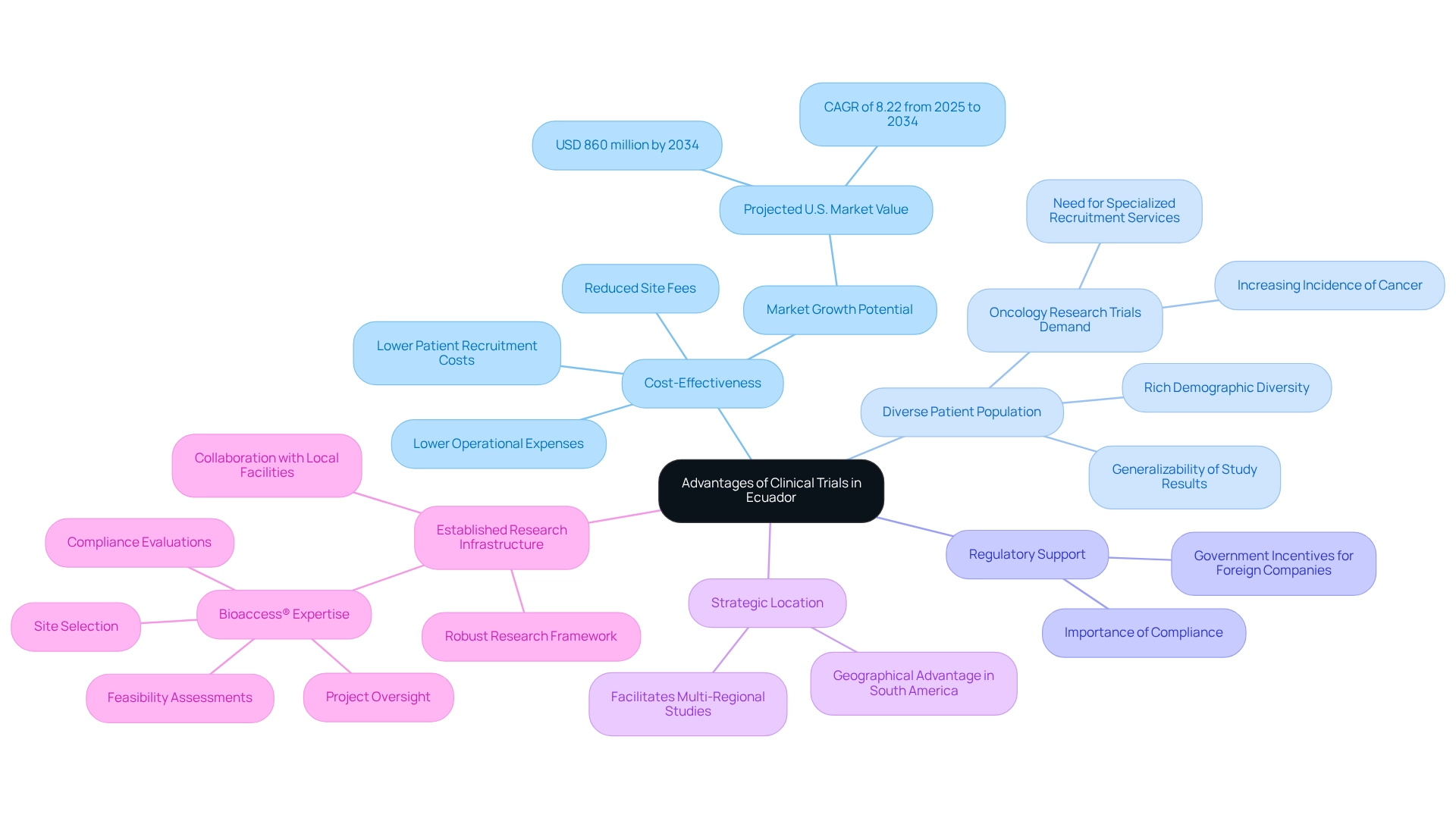
Leverage Ecuador's Advantages for Clinical Trials
To fully leverage the advantages of conducting clinical studies in Ecuador, consider the following key factors:
-
Cost-Effectiveness: Clinical studies in Ecuador offer significant cost savings compared to North America and Europe. Lower patient recruitment costs, site fees, and operational expenses contribute to a more budget-friendly research environment, making it an appealing option for Medtech companies. The U.S. market is projected to reach approximately USD 860 million by 2034, expanding at a CAGR of 8.22% from 2025 to 2034, indicating substantial growth opportunities for firms engaged in research activities in this region.
-
Diverse Patient Population: The rich demographic diversity in Ecuador enables the recruitment of a wide array of participants, enhancing the generalizability of study results. This diversity is particularly beneficial for studies targeting chronic diseases or conditions prevalent in specific populations, ensuring that findings are applicable across various demographics. The increasing incidence of cancer, for instance, is driving the demand for specialized patient recruitment services, as highlighted in the case study on oncology research trials.
-
Regulatory Support: The Ecuadorian government is increasingly supportive of clinical research, providing incentives for foreign companies to conduct studies. Staying informed about the latest regulations and guidelines is crucial for compliance and can help you capitalize on available support, streamlining the process.
-
Strategic Location: The country's geographical position in South America serves as a strategic hub for accessing other Latin American markets. This facilitates multi-regional studies, allowing for a broader research scope and the potential to engage diverse patient populations across the continent.
-
Established Research Infrastructure: Ecuador boasts a robust research framework, with numerous organizations and healthcare centers ready to conduct experimental studies. Collaborating with local medical facilities and academic institutions not only enhances the credibility of your research but also improves operational efficiency, ensuring that experiments are conducted seamlessly and effectively. With bioaccess®'s expertise in comprehensive research study management services—including feasibility assessments, site selection, compliance evaluations, and project oversight—Medtech companies can navigate the complexities of research with confidence. Furthermore, the impact of these studies on regional economies, such as job creation and healthcare improvement, underscores the importance of global collaboration in advancing medical technology, with a particular focus on building medtech partnerships in Ecuador, allowing medical technology firms to refine their clinical trial strategies and facilitate successful product development and market entry.
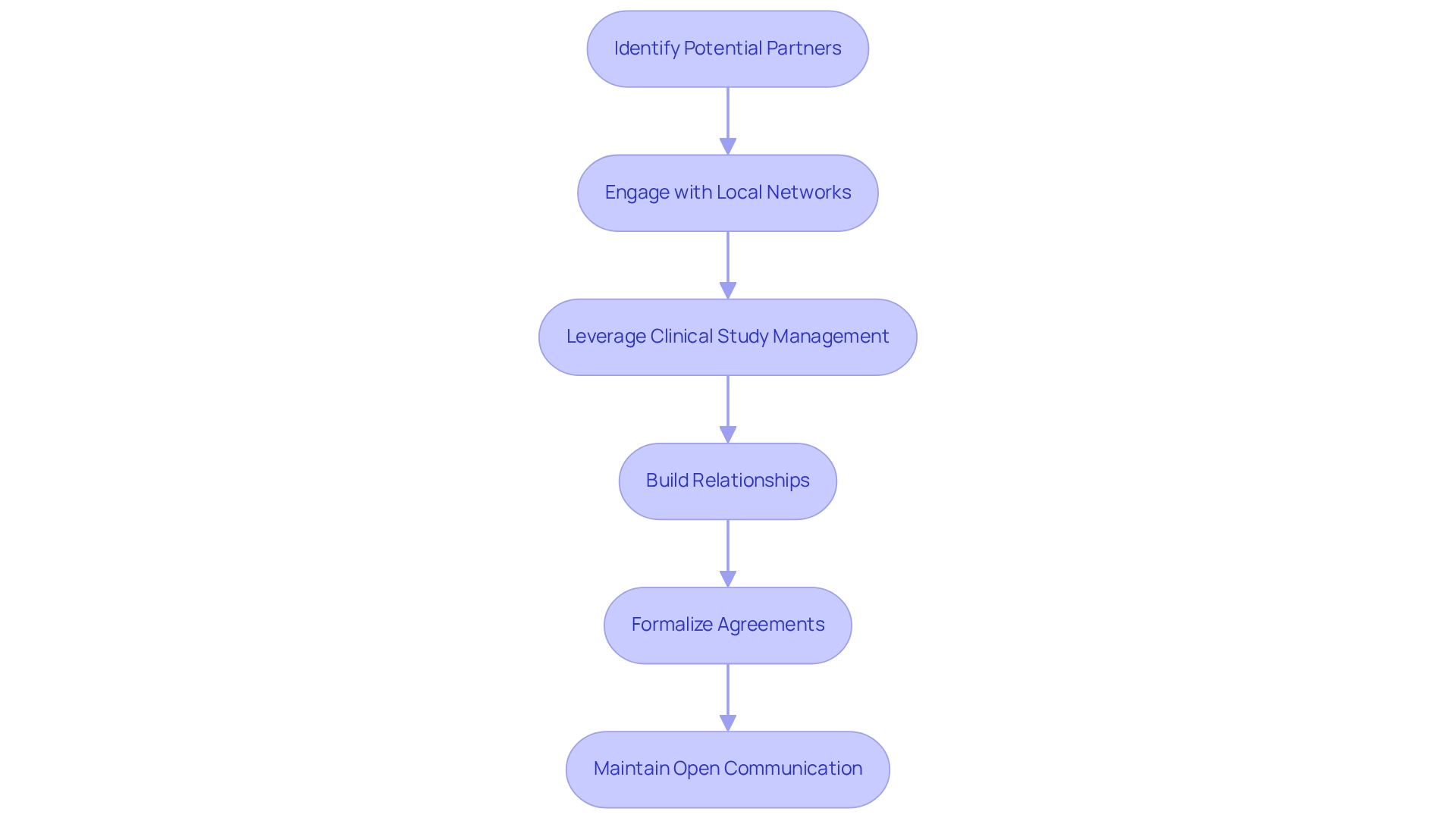
Establish Strategic Partnerships with Local Institutions
To effectively establish building medtech partnerships in Ecuador's medical technology landscape, consider the following steps:
- Identify Potential Partners: Seek out local universities, research institutions, and healthcare providers that align with your medical technology objectives. Evaluate their expertise, reputation, and prior involvement in clinical research to ensure compatibility.
- Engage with Local Networks: Actively participate in Medtech conferences, workshops, and networking events to connect with potential collaborators. Organizations such as Transform Health Ecuador offer valuable opportunities for networking and relationship building.
- Leverage Comprehensive Clinical Study Management Services: Collaborate with bioaccess to utilize their extensive capabilities, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. These services can simplify the research study process and improve the effectiveness of your collaborations. Furthermore, manage the ethics committee approval and nationalization of investigational devices as part of the study preparation.
- Build Relationships: Approach potential partners with a compelling value proposition. Clearly articulate how the collaboration can be mutually beneficial, whether through resource sharing, knowledge exchange, or access to new markets.
- Formalize Agreements: After identifying suitable partners, draft formal agreements that outline the terms of collaboration, including roles, responsibilities, and expectations. Ensure compliance with local laws and regulations to facilitate a smooth partnership.
- Maintain Open Communication: Cultivate ongoing communication with your partners to foster trust and effectively address any challenges that may arise. Regular meetings and updates are essential for keeping all parties aligned and engaged.
In 2025, as Ecuadorian healthcare providers adapt to evolving patient needs amidst economic fluctuations, the importance of strategic partnerships becomes even more pronounced. Building medtech partnerships in Ecuador through collaborations with local institutions can enhance the effectiveness of clinical trials and accelerate the development of innovative medical technologies. By utilizing local knowledge and resources, medical technology firms can maneuver through the intricacies of the Ecuadorian market and aid in the progress of healthcare solutions.
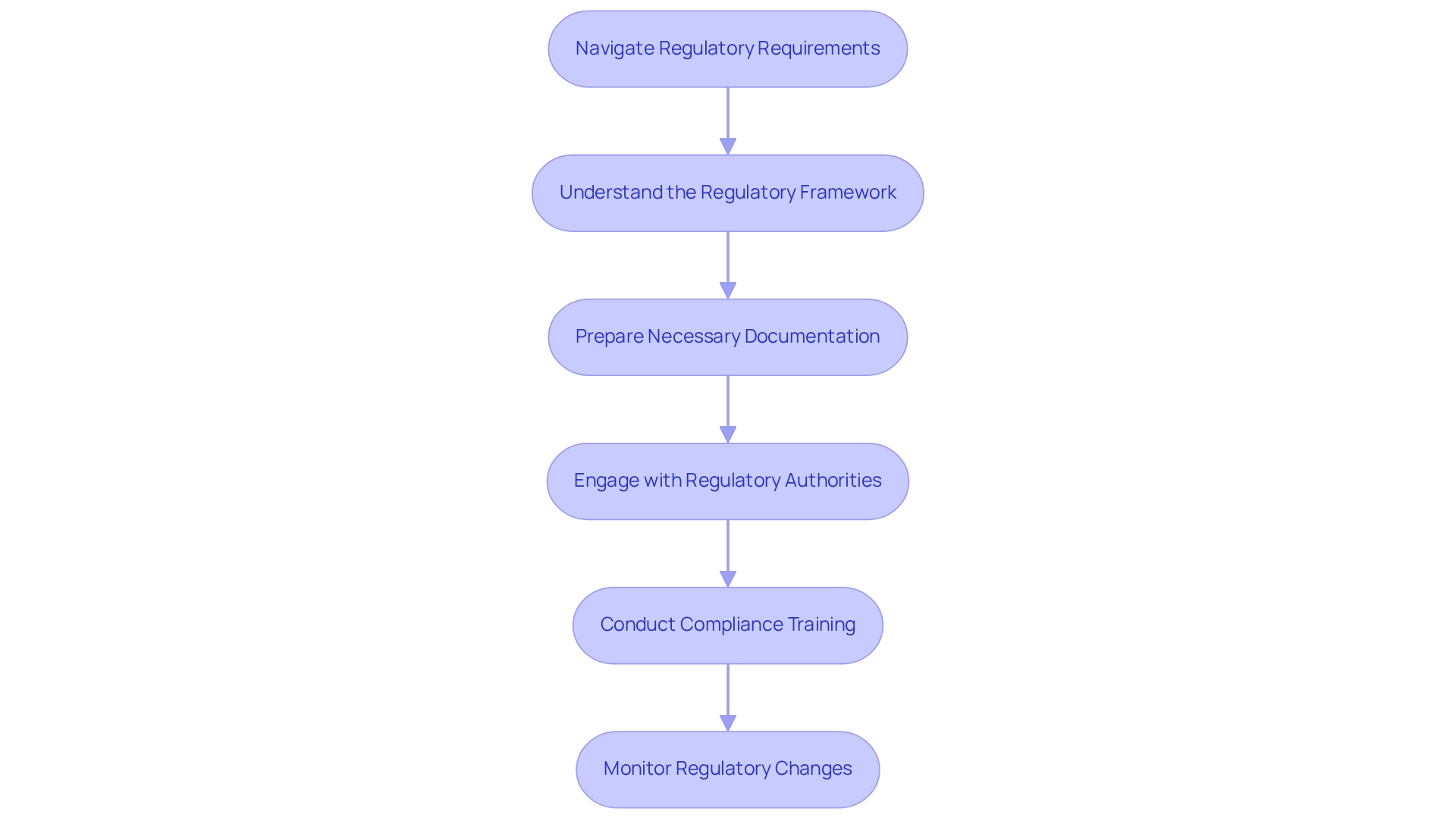
Navigate Regulatory Requirements and Compliance
For Medtech companies aiming to succeed in this dynamic market, building medtech partnerships in Ecuador while navigating the regulatory requirements is crucial. Here are key guidelines to follow:
-
Understand the Regulatory Framework: Gain a thorough understanding of the regulations governing medical devices and research studies in Ecuador, particularly those established by ARCSA. Familiarize yourself with the endorsement procedure for research studies and product registrations, as these are vital for adherence.
-
Prepare Necessary Documentation: Ensure that all required documentation is meticulously prepared and submitted according to local regulations. This includes clinical trial protocols, informed consent forms, and detailed product specifications, which are essential for a smooth approval process.
-
Engage with Regulatory Authorities: Build a strong relationship with ARCSA and other relevant regulatory bodies. Effective communication with these authorities can facilitate the approval process and keep you informed about any regulatory changes that may affect your operations.
-
Conduct Compliance Training: Implement training programs for your team focused on local regulatory requirements and compliance best practices. This training is imperative to ensure that all team members understand their roles and the significance of adhering to regulations, ultimately reducing the risk of non-compliance.
-
Monitor Regulatory Changes: Stay vigilant regarding any changes in the regulatory landscape that could impact your operations. Consistently subscribe to industry newsletters and work with local regulatory consultants to stay informed about the latest developments.
As of June 12, 2025, significant amendments to the Sanitary Technical Regulation of the country will come into effect, modernizing certification procedures and enhancing operational requirements for medical devices. This demonstrates the nation's dedication to improving its regulatory framework, making building medtech partnerships in Ecuador essential for medical technology firms to quickly adjust to these alterations. Notably, medicines that remain unmarketed in the country for one year will have their health registration suspended, underscoring the urgency of compliance and timely market entry. Additionally, Dr. Leopoldo Izquieta Pérez has noted that for medicines classified as 'over-the-counter,' the physical patient information leaflet may be substituted with a digital version once ARCSA establishes the website serving as a repository for approved leaflets. This emphasizes the changing environment of regulatory adjustments in which Medtech firms must maneuver efficiently.
At bioaccess®, we provide extensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. Our expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies ensures that your clinical trials are conducted efficiently and in compliance with local regulations. With a dedicated team led by professionals like Juan Cuya, MD, and Ana Criado, we are well-equipped to guide you through the complexities of the regulatory environment in Ecuador.
Conclusion
Ecuador's Medtech landscape presents a unique blend of challenges and opportunities, underscoring the importance of a strategic approach for companies seeking to establish a foothold in this burgeoning market. A thorough understanding of market dynamics, regulatory requirements, local healthcare needs, and the competitive landscape is essential for success. By leveraging the country’s advantages—such as cost-effective clinical trials and a diverse patient population—companies can position themselves favorably for growth and innovation.
Establishing strong partnerships with local institutions can further enhance the effectiveness of Medtech initiatives. These collaborations not only facilitate smoother clinical trial processes but also contribute to the overall improvement of healthcare solutions in Ecuador. Engaging with local stakeholders and understanding cultural nuances will pave the way for greater acceptance of new technologies, ensuring that innovations are not only introduced but also embraced by the community.
As the Medtech sector in Ecuador continues to evolve, navigating the regulatory landscape remains a critical factor. Staying informed about changes and maintaining compliance will be key to successful market entry and sustained operations. Companies that are well-prepared to adapt to these developments will undoubtedly lead the charge in transforming healthcare solutions for the local population, ultimately making a lasting impact on the industry and the communities it serves.
Frequently Asked Questions
What is the current state of the medical technology market in Ecuador?
The medical technology sector in Ecuador is projected to experience significant growth, particularly in nephrology and urology devices, with revenues expected to rise steadily from 2021 to 2031. The market is forecasted to reach $X million by 2031, reflecting a compound annual growth rate (CAGR) of Y%.
What regulatory body governs medical devices and clinical trials in Ecuador?
The primary regulatory body in Ecuador is the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA). It is crucial to familiarize yourself with their regulations to ensure compliance and successful market entry.
How can understanding local healthcare needs benefit Medtech partnerships in Ecuador?
Identifying specific healthcare needs, prevalent diseases, and technological gaps in the Ecuadorian population allows for the tailoring of Medtech solutions. This understanding can lead to better healthcare access and improved treatment outcomes, especially with the rising interest in artificial intelligence and machine learning in healthcare.
Who are the major competitors in the Ecuadorian Medtech market?
Major global players such as Medtronic and Johnson & Johnson are among the top three largest medical technology companies globally, highlighting the competitive nature of the market. Analyzing existing competitors helps to pinpoint market gaps and identify opportunities for differentiation.
What role do cultural considerations play in the acceptance of new healthcare technologies in Ecuador?
Cultural elements can significantly affect healthcare provision and the acceptance of new technologies. Engaging with local stakeholders to understand customs and practices is essential for ensuring that Medtech innovations are well-received and effectively utilized in the healthcare ecosystem.




