Overview
The benefits of US-Latin American MedTech collaboration include enhanced innovation, improved healthcare outcomes, and access to new markets, driven by the strengths of both regions in addressing local healthcare challenges. The article supports this by highlighting successful partnerships, such as the launch of the Celbrea® medical device in Colombia and the role of bioaccess™ in facilitating clinical trials, which collectively demonstrate how collaboration leads to tailored solutions and significant advancements in medical technology.
Introduction
In recent years, the collaboration between the United States and Latin American countries in the MedTech sector has emerged as a critical driver of innovation and healthcare advancement. This partnership uniquely integrates the technological prowess and funding capabilities of the US with the distinct healthcare challenges and emerging markets of Latin America.
As illustrated by successful initiatives like Welwaze Medical Inc.'s collaboration in Colombia, these alliances not only facilitate the development of tailored medical technologies but also enhance healthcare delivery in underserved regions. The increasing significance of these collaborations underscores a transformative shift in how healthcare challenges are addressed, paving the way for improved patient outcomes and economic growth.
As stakeholders navigate the complexities of regulatory environments and cultural differences, the potential for groundbreaking advancements in medical technology continues to expand, promising a future where both regions benefit from shared expertise and innovation.
The Importance of US-Latin American MedTech Collaboration
In recent years, the partnership between the US and South American nations in the MedTech sector has underscored the benefits of US-Latin American MedTech collaboration, making it increasingly essential. This collaboration showcases the benefits of US-Latin American medtech collaboration by harnessing the strengths of both areas: the US contributes cutting-edge technology and substantial funding, while Latin America offers emerging markets along with distinctive healthcare challenges that drive innovation. Noteworthy examples include:
- Welwaze Medical Inc.'s collaboration with bioaccess™ for the launch of the Celbrea® medical device in Colombia.
- ReGelTec's early feasibility study on HYDRAFIL™ for treating chronic low back pain.
Additionally, bioaccess™, as the sole vetted CRO and consulting partner for U.S. medical device companies in Colombia, plays a pivotal role in positioning Barranquilla as a leading destination for clinical trials, supported by Colombia's Minister of Health, enhancing the area's research capabilities. By working collaboratively, stakeholders can develop tailored medical technologies that address local needs and improve overall healthcare systems, showcasing the benefits of US-Latin American medtech collaboration. This synergy promotes knowledge exchange and showcases the benefits of US-Latin American medtech collaboration, allowing both regions to learn from each other's regulatory frameworks and patient care practices.
The trade balance of health equipment in Latin America and the Caribbean has been actively monitored from 2018 to 2023, highlighting the increasing significance of these partnerships. Experts assert that 'Latin American medical research and development transforms the medical sector,' emphasizing the benefits of US-Latin American medtech collaboration. Furthermore, a case study on nanotech innovation reveals how advancements in treating coronary diseases demonstrate the benefits of such partnerships, showcasing significant improvements in treatment methodologies.
The partnership is not just advantageous but revolutionary, showcasing the benefits of US-Latin American medtech collaboration and the continuous advancement of medical services in both areas.
Key Benefits of Collaborative Efforts in MedTech
Collaborative efforts within the MedTech sector present several compelling advantages that drive both innovation and enhanced medical service delivery, including:
- Enhanced Innovation: By leveraging the strengths of both US and South American companies, such as the collaboration between Welwaze Medical Inc. and bioaccess™ for the Celbrea® medical device launch in Colombia, these partnerships highlight the benefits of US-Latin American medtech collaboration in fostering the development of innovative medical technologies tailored to meet the needs of diverse patient populations. This synergy not only accelerates the development process but also ensures that the solutions are culturally and contextually appropriate.
- Improved Healthcare Outcomes: Collaborative initiatives frequently focus on addressing local health challenges, as demonstrated by the bioaccess™ partnership that supports various clinical trials, highlighting the benefits of US-Latin American medtech collaboration in significantly enhancing patient care in underserved areas. Such targeted approaches lead to tangible improvements in health metrics and overall community well-being, exemplified by the positive results shared by Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, during its first human trial in Colombia.
- Cost-Effectiveness: Partnerships facilitate shared investment in research and development, which can mitigate the financial burden associated with bringing new technologies to market. This collaborative approach not only encourages innovation but also ensures that providers can access state-of-the-art solutions without prohibitive costs.
- Access to New Markets: The benefits of US-Latin American medtech collaboration provide US companies with the opportunity to expand into the burgeoning Latin American markets, while local enterprises benefit from the infusion of advanced technologies and expertise. This reciprocal access creates a win-win scenario, enhancing the competitive landscape and driving economic growth across regions, which showcases the benefits of US-Latin American medtech collaboration.
- Expedited Commercialization and Regulatory Compliance: Collaborations, such as those with medical providers and organizations like bioaccess™, can significantly expedite the commercialization process and facilitate adherence to regulatory standards, highlighting the benefits of US-Latin American medtech collaboration for the successful introduction of new technologies. The experience shared by Dushyanth Surakanti exemplifies the positive impact of such partnerships.
The challenges faced by the MedTech industry are underscored by a statistic indicating that 88% of medical technology executives rated advances in technology as a top challenge. This emphasizes the necessity for teamwork to navigate these complexities effectively. Arthur D. Little emphasizes that 'Collaboration between different stakeholders is crucial to ensure that the devices can gain regulatory approval to achieve go-to-market timelines.' This insight underscores the foundational role that partnerships play in navigating the complex regulatory landscape, ultimately leading to successful healthcare outcomes.
A case study titled 'Challenges in Medical Technology' illustrates these points, revealing that executives face significant hurdles such as rapid technological advancements and regulatory changes. The result of this case study emphasizes the need for adaptability and teamwork in the industry.
Challenges in US-Latin American MedTech Partnerships
Despite the potential benefits of US-Latin American MedTech collaboration, several challenges must be navigated effectively.
-
Regulatory Differences: The landscape of medical technology regulation varies significantly between the United States and countries in South America. INVIMA, as the Colombia National Food and Drug Surveillance Institute, plays a critical role in this landscape, overseeing the marketing and manufacturing of health products and ensuring compliance with health standards.
Its classification as a Level 4 health authority by PAHO/WHO underscores its competence and efficiency in health regulation, which is essential for navigating these regulatory differences for successful market entry. Specifically, INVIMA's Directorate for Medical Devices and other Technologies is responsible for monitoring medical devices, suggesting technical standards, and ensuring quality assurance, which are vital for fostering a compliant environment for MedTech innovations.
-
Cultural Barriers: Distinct business practices, communication styles, and medical priorities can lead to misunderstandings and misalignments between US and South American partners.
Fostering mutual understanding and respect for local customs is essential to bridge these gaps and enhance collaboration.
-
Funding Limitations: While US firms typically enjoy access to extensive funding resources, their counterparts in South America often encounter financial constraints that can inhibit their participation in joint ventures.
This disparity can limit the scope of collaborative projects and affect overall project viability.
-
Logistical Issues: Geographic distances and varying medical infrastructures present logistical challenges in executing research and implementing technologies.
These issues can complicate patient recruitment and retention, particularly in the context of clinical trials. Notably, dropout rates in clinical trials in South America are significantly lower, at one-third of those in the U.S. and EU, largely due to the area's urbanization where over 80% of the 650 million individuals reside in urban environments, facilitating patient recruitment and retention.
Furthermore, the economic impact of MedTech clinical studies is substantial, contributing to job creation, enhancing healthcare systems, and fostering international collaboration. Julio G. Martinez-Clark, co-founder and CEO of bioaccess, emphasizes the potential of the area by stating,
Thanks to the efforts made by the different actors in the health sector, Colombia is a powerhouse in clinical research in Latin America.
This highlights the importance of overcoming these challenges to harness the full potential of MedTech innovations in the region and maximize the benefits of US-Latin American MedTech collaboration.
With the United States anticipated to produce US$221.00bn in the Medical Technology sector by 2025, the financial motivations for partnership are substantial, further highlighting the necessity for effective clinical trial management services to navigate the complexities of regulatory compliance and project execution.
Successful Case Studies in MedTech Collaboration
Numerous successful case studies illustrate the considerable benefits of US-Latin American MedTech collaboration.
Telemedicine Initiatives: One notable example involves a US-based telemedicine company that partnered with a leading Latin American health organization to implement remote medical solutions. This partnership significantly improved access to medical services for rural communities, leading to better patient outcomes and considerable decreases in medical expenses.
The rapid advancement of telemedicine initiatives in the area signifies a significant shift toward more accessible healthcare services.
Medical Device Development: A prime illustration of innovation tailored to specific health needs is the partnership between Welwaze Medical Inc. and bioaccess™ for the launch of the Celbrea® medical device in Colombia. This partnership not only navigated regulatory access but also showed a commitment to enhancing breast disease detection rates in the area, emphasizing how such alliances address urgent health requirements while promoting economic growth through job creation.
Bioaccess™ provided essential services including regulatory approval assistance, project management, and compliance reviews, ensuring a smooth entry into the Colombian market.
- Research Collaborations: In academia, universities throughout the US and South America have established partnerships on various research projects. These partnerships have produced substantial advancements in treatment protocols and clinical trial methodologies, ultimately aiding medical systems in both regions.
Furthermore, experts agree that dropout rates in Latin America are one-third of those in the U.S. and the EU, highlighting the importance of strategic partnerships to overcome these challenges. Julio Martinez-Clark, CEO and co-Founder of Bioaccess, emphasizes this trend, stating,
Countries like Colombia are really, really making an effort to bring business, to bring investment and to bring science technology and innovation to the country.
This sentiment shows an increasing awareness of the significance of cross-border teamwork in improving medical solutions.
Bioaccess exemplifies this partnership, serving as a contract research organization that supports MedTech companies by navigating regulatory approval and patient recruitment in a cost-effective environment. This enables more comprehensive studies without sacrificing quality, ultimately aiding successful market access.
These examples highlight the significant influence of US-Latin American MedTech partnerships, illustrating the benefits of US-Latin American MedTech collaboration in creating innovative solutions to tackle critical healthcare challenges.
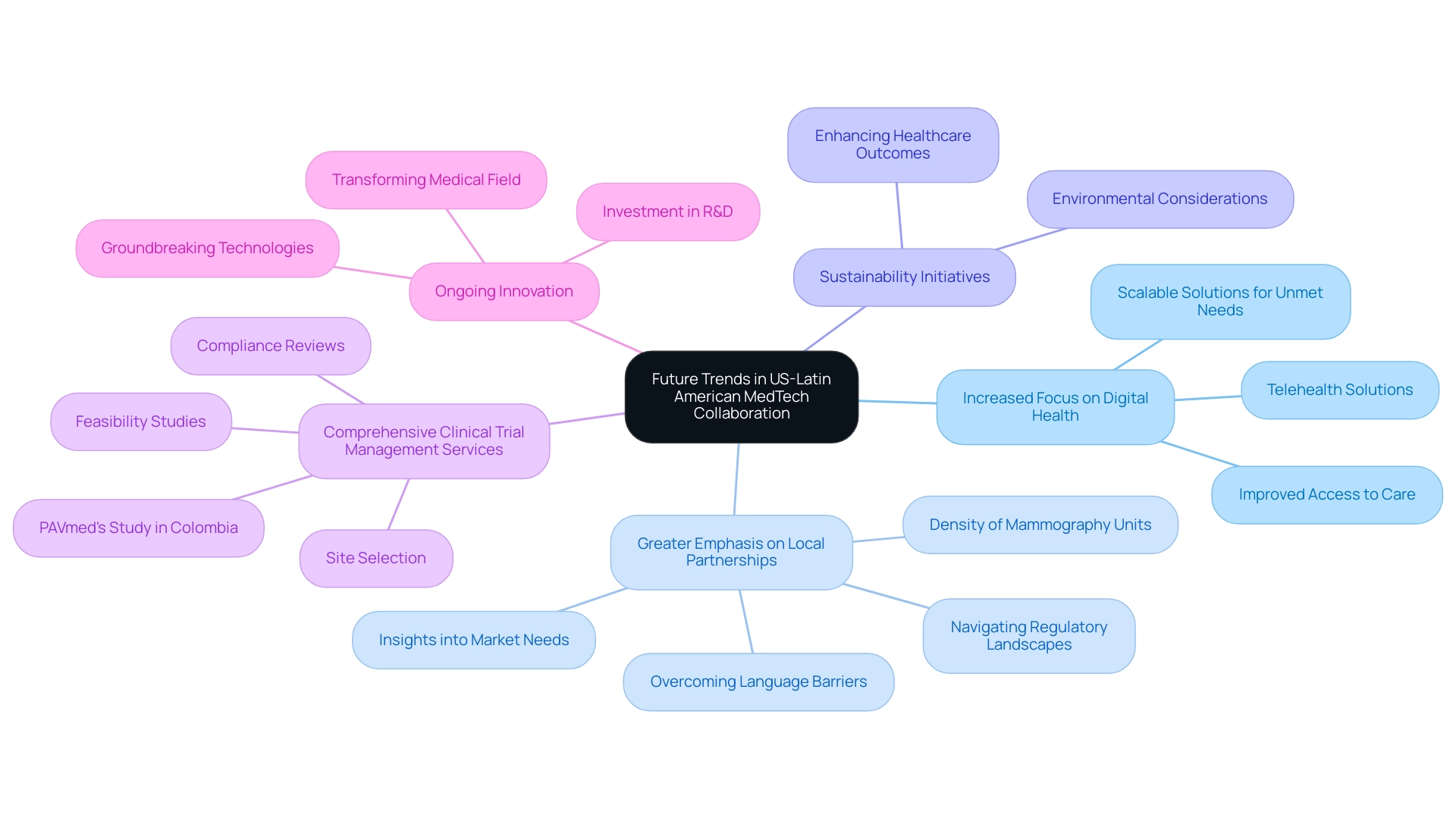
Future Trends in US-Latin American MedTech Collaboration
As we look ahead, several critical trends are poised to define the landscape of US-Latin American MedTech partnerships in 2024 and beyond:
- Increased Focus on Digital Health: The surge in telehealth and digital health solutions is set to open new avenues for partnership. With Medicare representing 21% of total medical expenditure in 2021, these innovations not only promise to improve access to care but also provide scalable solutions to tackle the unique challenges encountered by both regions, unlocking new markets and addressing unmet medical needs.
- Greater Emphasis on Local Partnerships: US companies are increasingly recognizing the value of forging partnerships with local firms. Such collaborations can facilitate navigation through regulatory landscapes and provide deeper insights into market needs, ultimately leading to a more integrated and effective approach to healthcare delivery. This is especially crucial given the challenges of language barriers, resource fragmentation, and regulatory hurdles in Latin America. For instance, understanding the density of mammography units in the region can guide targeted initiatives to improve screening access and outcomes. Successful partnerships, such as those between US Medtech companies and local providers, have demonstrated the benefits of US-Latin American Medtech collaboration in overcoming these barriers through shared expertise.
- Sustainability Initiatives: A growing emphasis on sustainability will shape collaborative efforts in the MedTech sector. By developing solutions that prioritize environmental considerations, stakeholders can address pressing ecological concerns while simultaneously enhancing healthcare outcomes.
- Comprehensive Clinical Trial Management Services: To successfully bridge the gap between innovation and execution, Medtech companies must leverage comprehensive services such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These services play a crucial role in navigating the complexities of clinical trials in South America, highlighted by successful examples like PAVmed's first-in-human study in Colombia, which overcame regulatory hurdles and demonstrated the potential for local economic impact.
- Ongoing Innovation: As investment in research and development continues to rise, the partnership between the US and South America is anticipated to produce groundbreaking technologies, showcasing the benefits of US-Latin American medtech collaboration. Latin American medical research and development is transforming the medical field, positioning the area as a hotspot for innovations that can change lives on a global scale. These advancements are likely to significantly improve patient outcomes and enhance healthcare efficiency, establishing the region as a burgeoning hub for medical innovation.
With the focus on digital health remaining vital, stakeholders are encouraged to leverage these trends to foster impactful collaborations that address both regional and global healthcare challenges.
Conclusion
The collaboration between the United States and Latin American countries in the MedTech sector represents a transformative approach to addressing healthcare challenges and driving innovation. By harnessing the technological advancements and funding capabilities of the US alongside the unique healthcare needs and emerging markets of Latin America, these partnerships have shown significant potential in enhancing healthcare delivery. Examples such as the successful launch of the Celbrea® medical device in Colombia and advancements in telemedicine illustrate the tangible benefits of these collaborative efforts.
Despite the challenges of regulatory differences, cultural barriers, and funding limitations, the strategic alliances formed in the MedTech space have proven to be advantageous. The shared knowledge and resources foster a more effective response to local health issues, leading to improved patient outcomes and economic growth. As stakeholders navigate these complexities, the emphasis on local partnerships and comprehensive clinical trial management services will be crucial in overcoming obstacles and maximizing the impact of innovations.
Looking ahead, the future of US-Latin American MedTech collaboration is bright, with trends such as increased focus on digital health, sustainability initiatives, and continued investment in research and development set to shape the landscape. These collaborations not only promise to address unmet healthcare needs but also position both regions as leaders in medical technology innovation. The ongoing commitment to collaboration will undoubtedly pave the way for improved healthcare systems, ultimately benefiting communities across both the United States and Latin America.
Frequently Asked Questions
What are the main benefits of US-Latin American MedTech collaboration?
The main benefits include enhanced innovation, improved healthcare outcomes, cost-effectiveness, access to new markets, and expedited commercialization and regulatory compliance.
How does US-Latin American collaboration enhance innovation in MedTech?
By leveraging the strengths of both US and South American companies, such collaborations foster the development of innovative medical technologies tailored to meet diverse patient needs, ensuring solutions are culturally and contextually appropriate.
Can you provide an example of successful collaboration in the MedTech sector?
An example is the collaboration between Welwaze Medical Inc. and bioaccess™ for the launch of the Celbrea® medical device in Colombia, which highlights the benefits of US-Latin American MedTech collaboration.
How does collaboration improve healthcare outcomes in Latin America?
Collaborative initiatives focus on local health challenges, leading to tangible improvements in health metrics and overall community well-being, as seen in the positive results from Sparta Biomedical's first human trial in Colombia.
In what way is collaboration cost-effective for MedTech companies?
Partnerships facilitate shared investment in research and development, which helps mitigate the financial burden of bringing new technologies to market, encouraging innovation without prohibitive costs.
What opportunities do US companies gain from collaborating with Latin American firms?
US companies gain access to burgeoning Latin American markets, while local enterprises benefit from advanced technologies and expertise, creating a win-win scenario that enhances economic growth.
How does collaboration expedite commercialization and regulatory compliance?
Collaborations with organizations like bioaccess™ can significantly speed up the commercialization process and help ensure adherence to regulatory standards, facilitating the successful introduction of new technologies.
What challenges does the MedTech industry face that necessitate collaboration?
The MedTech industry faces challenges such as rapid technological advancements and regulatory changes, with 88% of executives identifying these as top challenges, highlighting the need for teamwork to navigate complexities effectively.

