Overview
US-Latin American collaboration in MedTech is significant for enhancing healthcare innovations and improving patient outcomes through shared resources and expertise, despite facing challenges like regulatory hurdles and cultural differences. The article illustrates this importance by highlighting successful partnerships, such as Avantec Vascular's clinical study and Welwaze Medical's device launch, which demonstrate how these collaborations can lead to cost-effective innovation and expanded market access.
Introduction
The collaboration between the United States and Latin America in the MedTech sector is becoming increasingly vital as both regions strive to enhance healthcare delivery and patient outcomes. By uniting resources, expertise, and innovative ideas, these partnerships are paving the way for groundbreaking advancements in medical technologies.
Noteworthy initiatives, such as the clinical studies by Avantec Vascular and the successful launch of the Celbrea® medical device by Welwaze Medical Inc. in Colombia, exemplify the potential of these collaborations to navigate regulatory landscapes and address pressing healthcare needs.
However, challenges such as cultural differences, regulatory barriers, and resource allocation must be navigated to fully realize the benefits of this collaboration.
As the orthopedic devices market in Latin America continues to expand, the opportunities for innovation and improved healthcare access become ever more apparent, highlighting the significance of strategic partnerships in the MedTech industry.
The Significance of US-Latin American Collaboration in MedTech
The us-latin american collaboration in medtech is crucial for advancing healthcare innovations that improve patient care and outcomes. By merging resources, expertise, and innovative ideas, these partnerships create an environment that promotes research and development. A notable example is Avantec Vascular's first-in-human clinical study of an innovative vascular device in South America, supported by bioaccess™, which highlights the effective navigation of regulatory requirements and selection of principal investigators.
Additionally, Welwaze Medical Inc. collaborates with bioaccess™ for the launch of the Celbrea® medical device, demonstrating the importance of regulatory access in Colombia's market. However, US MedTech companies face significant challenges in South America, including:
- Language barriers
- Fragmented resources
- Regulatory hurdles
The orthopedic devices market in South America is projected to generate $3.6 billion in revenue from 2017 to 2029, underscoring the growing demand for advanced medical solutions and the potential for innovation through collaboration.
Such partnerships, particularly through us-latin american collaboration in medtech, not only address gaps in medical access but also facilitate the exchange of best practices and regulatory insights. As Ignacio Apella and Guillermo Montt from the International Labor Organization and World Bank Group state, 'The income protection system for the elderly in Paraguay exemplifies how contributions can improve coverage, equity, and sustainability.' This principle is relevant to us-latin american collaboration in medtech, as these collaborations can lead to customized solutions that address various healthcare requirements across regions, ultimately enhancing healthcare outcomes for populations in both the US and South America.
Key Benefits of US-Latin American MedTech Partnerships
The advantages of US-Latin American collaboration in medtech can significantly enhance the operational effectiveness of companies involved in these partnerships. Key benefits include:
- Access to Emerging Markets: These collaborations enable US firms to penetrate markets in South America, thereby expanding their reach and customer base. Recent trends indicate an increasing interest from US companies in US-Latin American collaboration in medtech to establish a foothold in these burgeoning markets. Comprehending the consumption trends of Generation Z in South America is crucial for tailoring products to meet the needs of this demographic.
- Cost-Effective Innovation: By pooling resources and expertise, partners can achieve substantial reductions in research and development costs. This collaborative approach not only accelerates the innovation cycle through US-Latin American collaboration in medtech but also makes advanced medical solutions more financially accessible. For instance, the recent statistic showing GPA's 80% growth in advertising revenues from retail media in 2023 illustrates the potential for financial success in these emerging markets.
- Diverse Perspectives: Working alongside professionals from various cultural and professional backgrounds fosters a rich environment for creativity and problem-solving. This diversity can lead to innovative product development that meets the distinct needs of American consumers.
- Regulatory Knowledge: Collaborations yield critical insights into navigating the complex landscape of local regulations, particularly through understanding INVIMA's role as the National Food and Drug Surveillance Institute in Colombia. As a Level 4 health authority recognized by PAHO/WHO, INVIMA ensures compliance with health standards, which is essential for US-Latin American collaboration in medtech and for medical technology companies looking to enter the Colombian market. The Directorate for Medical Devices and other Technologies within INVIMA specifically oversees the regulation of medical devices, suggesting technical standards for manufacturing, marketing, and quality assurance. This understanding facilitates smoother market entry and is crucial for the success of clinical studies, such as those conducted by bioaccess®, led by Dushyanth Surakanti, who stated, "bioaccess® exemplifies regulatory excellence in Latin America, ensuring that we meet all necessary standards for our clinical trials."
These benefits not only enhance the competitive advantage of the companies involved but also contribute significantly to healthcare advancements across both regions, highlighting the importance of US-Latin American collaboration in medtech for a more integrated and efficient medical technology ecosystem.
Challenges in US-Latin American MedTech Collaboration
Despite the numerous advantages that the US-Latin American collaboration in medtech can offer, several challenges persist, including:
-
Cultural Differences:
Variations in business practices and communication styles often result in misunderstandings and misaligned objectives. For instance, motivational factors can differ significantly across cultures, where some individuals may be driven by personal achievements, while others prioritize collaborative success and advancement opportunities.
Understanding these cultural nuances is crucial for effectively motivating a diverse workforce. To tackle these challenges, organizations can introduce webinars and organizational culture transformation packages that promote better communication and teamwork.
-
Regulatory Barriers:
The complexity of navigating differing regulatory environments, such as those overseen by INVIMA in Colombia, can pose significant hurdles. Established in 1992, INVIMA is responsible for the oversight of health products, including medical devices, and is categorized as a Level 4 health authority by PAHO/WHO, ensuring safety, efficacy, and quality. Within INVIMA, the Directorate for Medical Devices and other Technologies plays a crucial role in monitoring and controlling medical devices, suggesting technical standards, and overseeing pre- and post-market programs.
This regulatory framework is essential for fostering innovation and facilitating medical technology clinical studies that can lead to job creation and healthcare improvements in local economies.
-
Resource Allocation:
Disparities in resources and funding can create tension among partners, ultimately impacting the viability of projects and the achievement of shared goals.
-
Trust Issues:
Establishing trust between organizations from different regions is critical yet often complicated by historical disparities or past negative experiences. The Society for Human Resource Management (SHRM) indicates that organizations with diversity training programs experience a 65% rise in employee engagement and productivity, showing that tackling cultural challenges through proactive measures can result in more successful partnerships.
Furthermore, research shows that millennials are 83% more likely to be engaged at work in inclusive companies, highlighting the importance of fostering an inclusive environment.
Comprehending these challenges is crucial for creating effective solutions and nurturing successful US-Latin American collaboration in medtech within the medical technology sector. The case study titled 'Cultural Variations in Motivation' illustrates this well; organizations that recognize and adapt to varying motivational factors can tailor their approaches to better engage their diverse workforce, ultimately leading to improved teamwork and success.
Successful Case Studies in US-Latin American MedTech Collaboration
Numerous successful case studies illustrate the significant advantages of US-Latin American MedTech partnership:
- Telemedicine Initiatives: A significant partnership between a US-based telehealth provider and a Brazilian medical organization has markedly improved access to medical services in rural areas. This project exemplifies how innovative technology can effectively bridge medical gaps, particularly in underserved regions, thereby enhancing patient outcomes.
- Device Development: In a compelling joint venture, a US medical device company partnered with an Argentine manufacturer to create a cost-effective cardiac monitoring device specifically designed to meet local market requirements. This initiative not only emphasizes the potential for innovation through teamwork but also addresses the crucial need for affordable medical solutions, highlighting the importance of US-Latin American collaboration in MedTech.
- Regulatory and Market Access: Welwaze Medical Inc. has successfully collaborated with bioaccess™ as its regulatory and market access consulting firm to launch the Celbrea® medical device in Colombia. This partnership has streamlined the regulatory process, facilitating market access and demonstrating the effectiveness of local expertise in navigating complex healthcare environments. The partnership is a significant step towards enhancing early detection of breast disease in the region, which can lead to improved health outcomes and economic benefits.
- Research Studies: Collaborative clinical trials conducted between US researchers and institutions in South America have yielded substantial findings concerning tropical diseases. These partnerships have resulted in the creation of enhanced treatment protocols, showcasing the strength of US-Latin American collaboration in MedTech to advance medical research.
These case studies not only emphasize the successful outcomes of joint efforts but also provide valuable insights into the vast potential that exists within US-Latin American collaboration in MedTech. The orthopedic devices market in Latin America, valued at approximately 3.6 billion U.S. dollars, highlights the importance of these partnerships in accessing profitable opportunities. Additionally, the medical device markets in Chile and Colombia are projected to grow at CAGRs of 9% and 8.4%, respectively, driven by improving health systems and government support.
As mentioned by Anil Kumar P., a Research Manager in the medical field, whether collaborating with small start-ups or large enterprises, high-quality research supports informed decision-making and drives impactful results in the industry. This feeling strengthens the significance of promoting such partnerships to improve medical service delivery across areas. Furthermore, these partnerships create jobs, promote economic growth, and enhance healthcare, ultimately benefiting local economies.
To further support these efforts, a step-by-step method to encourage cooperation in the medical technology sector can offer practical guidance for stakeholders aiming to maximize these opportunities.
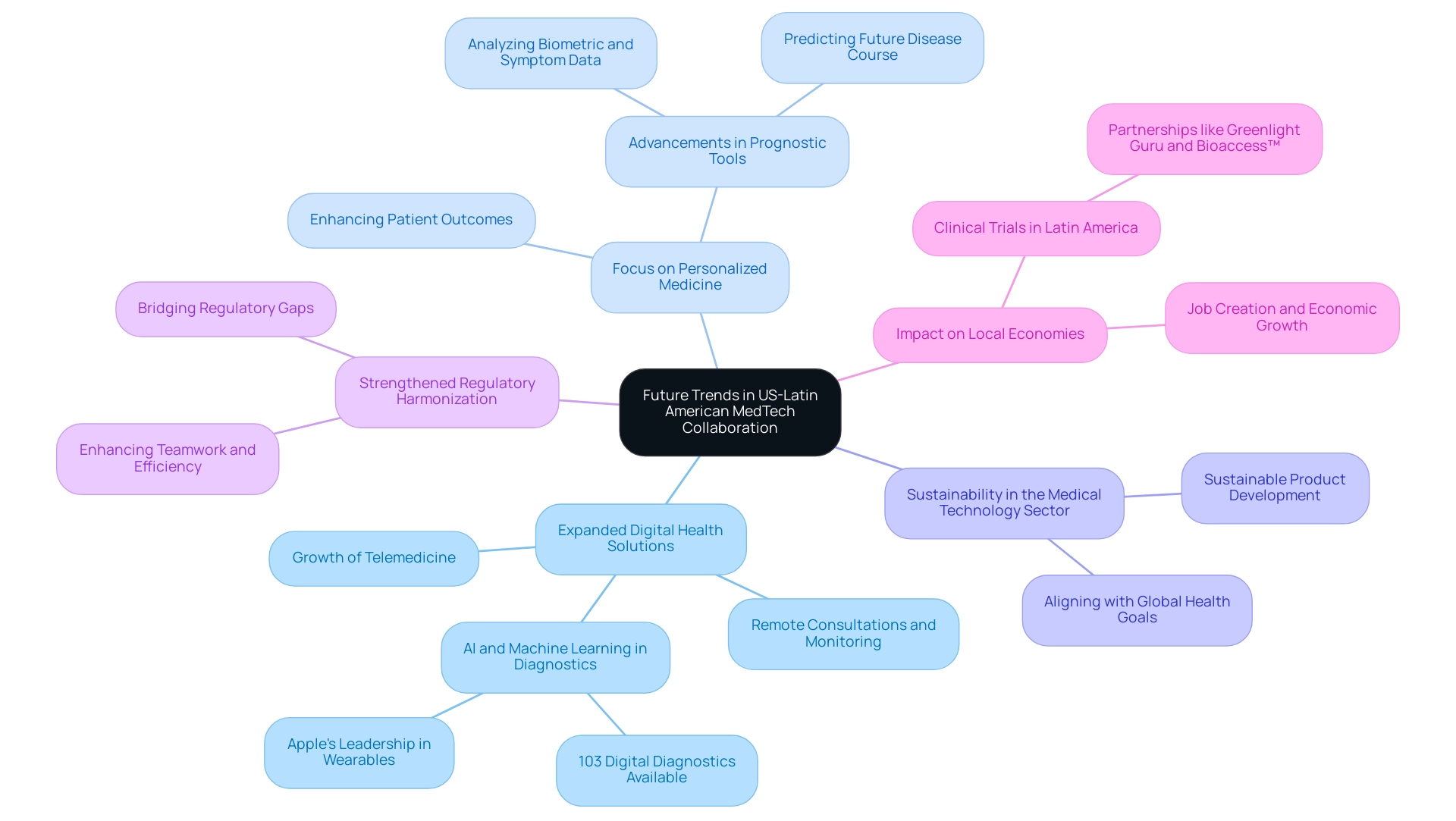
Future Trends in US-Latin American MedTech Collaboration
The landscape of US-Latin American collaboration in MedTech is on the brink of significant transformation. As we move into 2024, several key trends are emerging that will shape this sector:
- Expanded Digital Health Solutions: The growth of telemedicine and digital wellness platforms is poised to improve cooperation among medical providers internationally. More than 103 digital diagnostics for disease assessment are now commercially available, many leveraging artificial intelligence and machine learning. Significantly, Apple was the top wearable device supplier globally in 2022, demonstrating the increasing influence of digital health solutions on medical technology collaboration. This shift allows for remote consultations and continuous patient monitoring, drastically improving accessibility and efficiency.
- Focus on Personalized Medicine: There is a growing emphasis on developing personalized medical solutions tailored to specific populations. This trend is underpinned by advancements in prognostic tools that analyze biometric and symptom data to predict a patient's future disease course. These tools not only provide insights into therapy response but also alert healthcare providers to inform care decisions, ultimately enhancing patient outcomes. For instance, a case study on prognostic tools demonstrated that these digital devices can effectively predict a patient’s future course of disease, providing critical insights that inform care decisions.
- Sustainability in the Medical Technology Sector: Sustainability is becoming increasingly important within product development and manufacturing processes. Stakeholders are recognizing the need to align their practices with global health and environmental goals, ensuring that innovations in MedTech contribute positively to both health and the planet.
- Strengthened Regulatory Harmonization: Efforts to harmonize regulatory standards between the US and South America are gaining momentum. Challenges such as regulatory hurdles, language barriers, and fragmented resources faced by US MedTech companies underscore the necessity of US-Latin American collaboration in MedTech to bridge gaps in clinical research and innovation. This regulatory alignment will enhance teamwork efforts, making partnerships more efficient and effective.
- Impact on Local Economies: MedTech clinical studies are not only pivotal for advancing healthcare but also play a critical role in job creation, economic growth, and healthcare improvement in Latin America. As the partnership between firms like Greenlight Guru and Bioaccess™ accelerates innovations and clinical trials in regions such as Colombia, the positive outcomes on local economies become increasingly evident. Media coverage, such as that from Clinical Leader, highlights the progress and opportunities within this sector.
By staying informed about these trends, stakeholders can strategically position themselves for success in the rapidly evolving MedTech landscape. Dr. Wesley Smith highlights the significance of teamwork in the development of innovative solutions, stating, "Through research, I am dedicated to the betterment of muscle testing in the elderly and exploring new exercise strategies specifically designed to combat age-associated functional decline." This commitment to innovation and collaboration is pivotal as the sector continues to evolve.
Conclusion
The collaboration between the United States and Latin America in the MedTech sector presents a significant opportunity to improve healthcare delivery and patient outcomes. By merging resources, expertise, and innovative solutions, these partnerships address pressing healthcare needs while fostering the development of advanced medical technologies tailored to local markets. Successful initiatives, such as the launch of the Celbrea® medical device, demonstrate the effectiveness of navigating complex regulatory landscapes through collaboration.
However, challenges such as:
- Cultural differences
- Regulatory hurdles
- Resource allocation
must be addressed to fully realize the benefits of these partnerships. Overcoming these obstacles is essential for building trust and effective communication among stakeholders, which can enhance collaboration outcomes.
Looking ahead, trends like:
- Digital health solutions
- Personalized medicine
- Sustainability
will shape the future of US-Latin American MedTech collaboration. Efforts to harmonize regulatory standards will further streamline partnerships, ensuring that clinical studies contribute positively to local economies.
In conclusion, the potential for growth and innovation in the MedTech sector is substantial. Strategic partnerships between the US and Latin America are crucial to unlocking this potential, leading to enhanced healthcare access and economic development. A commitment to collaboration will be vital in navigating the evolving landscape of medical technology, ultimately benefiting patients and communities across both regions.
Frequently Asked Questions
Why is US-Latin American collaboration in medtech important?
This collaboration is crucial for advancing healthcare innovations that improve patient care and outcomes by merging resources, expertise, and innovative ideas, promoting research and development.
Can you provide an example of a successful collaboration in medtech?
A notable example is Avantec Vascular's first-in-human clinical study of an innovative vascular device in South America, supported by bioaccess™, which effectively navigated regulatory requirements and selected principal investigators.
What challenges do US MedTech companies face in South America?
US MedTech companies encounter significant challenges in South America, including language barriers, fragmented resources, and regulatory hurdles.
What is the projected revenue for the orthopedic devices market in South America?
The orthopedic devices market in South America is projected to generate $3.6 billion in revenue from 2017 to 2029, indicating a growing demand for advanced medical solutions.
How do US-Latin American collaborations benefit healthcare access?
These partnerships address gaps in medical access, facilitate the exchange of best practices, and provide regulatory insights, ultimately enhancing healthcare outcomes for populations in both the US and South America.
What are some advantages of US-Latin American collaboration in medtech?
Key benefits include access to emerging markets, cost-effective innovation, diverse perspectives, and regulatory knowledge, which enhance operational effectiveness and contribute to healthcare advancements.
How does collaboration help in understanding local market trends?
Collaborations enable US firms to penetrate South American markets and comprehend consumption trends, particularly among Generation Z, allowing them to tailor products to meet local needs.
What role does INVIMA play in the regulatory landscape for medtech in Colombia?
INVIMA is the National Food and Drug Surveillance Institute in Colombia, ensuring compliance with health standards as a Level 4 health authority recognized by PAHO/WHO, which is essential for navigating local regulations in medtech.
How do diverse perspectives contribute to product development in medtech?
Working alongside professionals from various cultural and professional backgrounds fosters creativity and problem-solving, leading to innovative product development that meets the distinct needs of consumers.
What is the impact of these collaborations on the financial aspects of medtech innovation?
By pooling resources and expertise, collaborations can achieve substantial reductions in research and development costs, accelerating the innovation cycle and making advanced medical solutions more financially accessible.

