Overview
Clinical trial innovation for Medtech in Chile is pivotal, requiring a thorough understanding of the regulatory landscape, strategic planning, and the effective execution of studies tailored to local health needs.
- A supportive regulatory environment
- A diverse patient population
- Comprehensive study management services
These factors collectively enhance the adaptability and ethical standards of Medtech research in the region, underscoring the critical role they play in addressing healthcare challenges.
Collaboration among stakeholders is not just beneficial; it is essential for advancing clinical research and ensuring that innovative solutions meet the needs of the population.
Introduction
Chile is emerging as a pivotal player in the realm of Medtech clinical trials, drawing attention for its robust healthcare framework and commitment to ethical research.
With over 1,000 healthcare facilities and a diverse population of approximately 20 million, the country offers a unique landscape for conducting clinical studies that are both representative and impactful.
As the number of clinical trial registrations continues to rise, understanding the intricacies of regulatory compliance and strategic planning becomes essential for researchers aiming to navigate this complex environment.
From the initial protocol development to post-trial data analysis, each phase presents opportunities and challenges that can significantly influence the success of Medtech innovations.
By exploring best practices and leveraging local expertise, stakeholders can enhance their contributions to advancing healthcare solutions that address the specific needs of the Chilean population.
Understand the Landscape of Medtech Clinical Trials in Chile
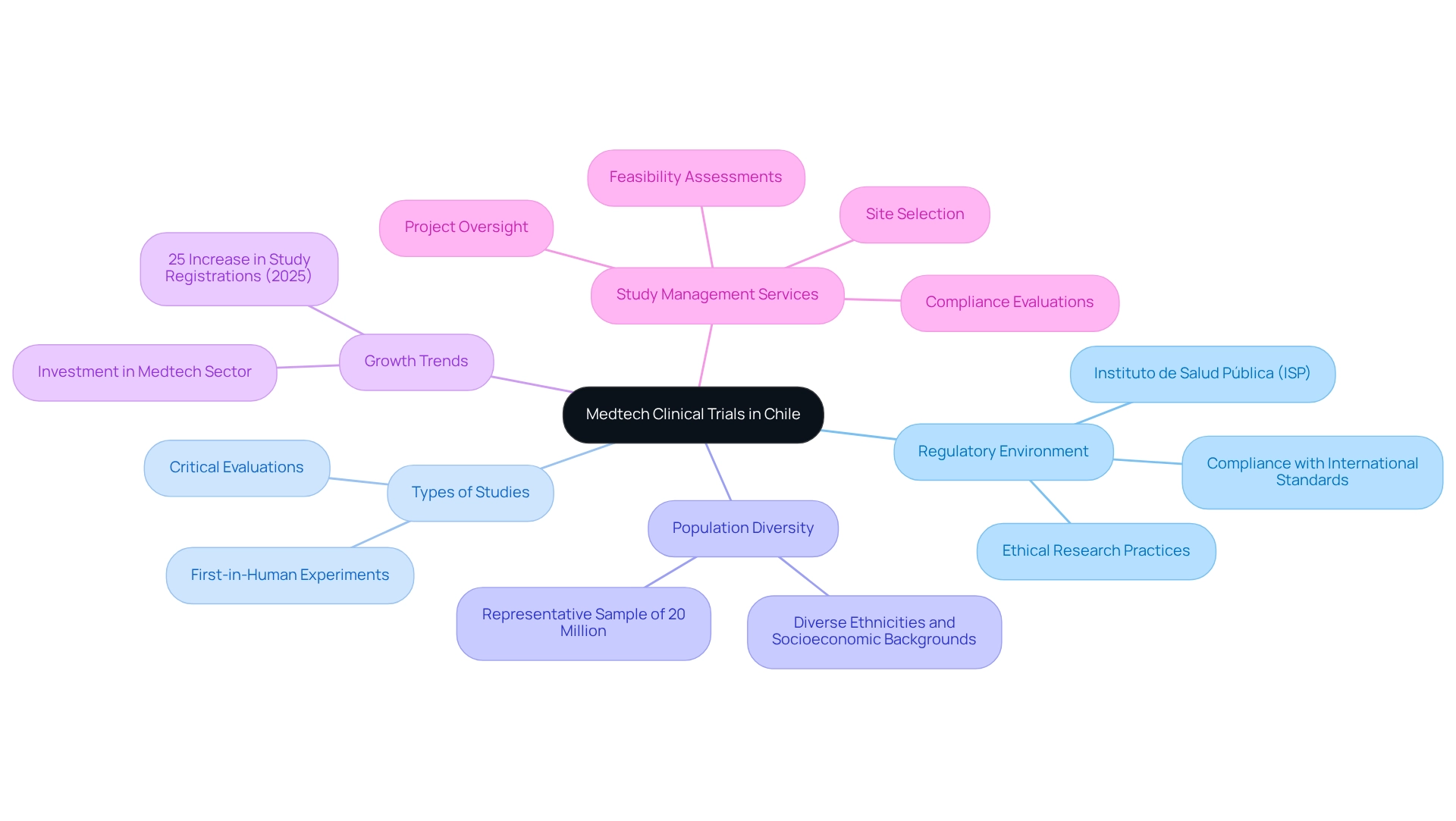
To effectively carry out medical research in Chile, it is essential to comprehend the evolving landscape of Medtech studies. Chile has established itself as a leading center for medical research in Latin America, bolstered by a robust healthcare infrastructure that includes over 1,000 healthcare facilities and a high level of medical expertise. This efficient regulatory process, overseen by the Instituto de Salud Pública (ISP), ensures compliance with international standards and promotes ethical research practices, thereby enhancing public confidence in the process.
Familiarity with the various types of examinations typically conducted, such as first-in-human experiments and critical evaluations, is vital. The diverse population of approximately 20 million, encompassing various ethnicities and socioeconomic backgrounds, provides a representative sample that enhances the applicability of research findings. For instance, the NeuroStim experiment revealed that 70% of participants experienced significant reductions in depression symptoms, illustrating the potential impact of well-structured research within this demographic. This diversity aligns with the necessity for studies to be adaptable to local health issues, as underscored by recent research.
Recent trends indicate a notable increase in study registrations in Chile, with a reported 25% rise in 2025 compared to the previous year, signaling a growing investment in the Medtech sector. This surge reflects the country's commitment to tackling local health challenges through innovative solutions. By leveraging the comprehensive study management services provided by bioaccess®, which include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting on serious and non-serious adverse events, researchers can effectively navigate the complexities of the regulatory environment. Understanding these insights, coupled with the unique demographic and geographic advantages of Chile, enables researchers to design effective studies that not only meet regulatory standards but also address the health needs of the population. Ultimately, the combination of a supportive regulatory environment, a diverse patient population, and a commitment to ethical standards positions Chile as an ideal location for advancing Medtech innovations.
Prepare for Clinical Trials: Regulatory Compliance and Strategic Planning
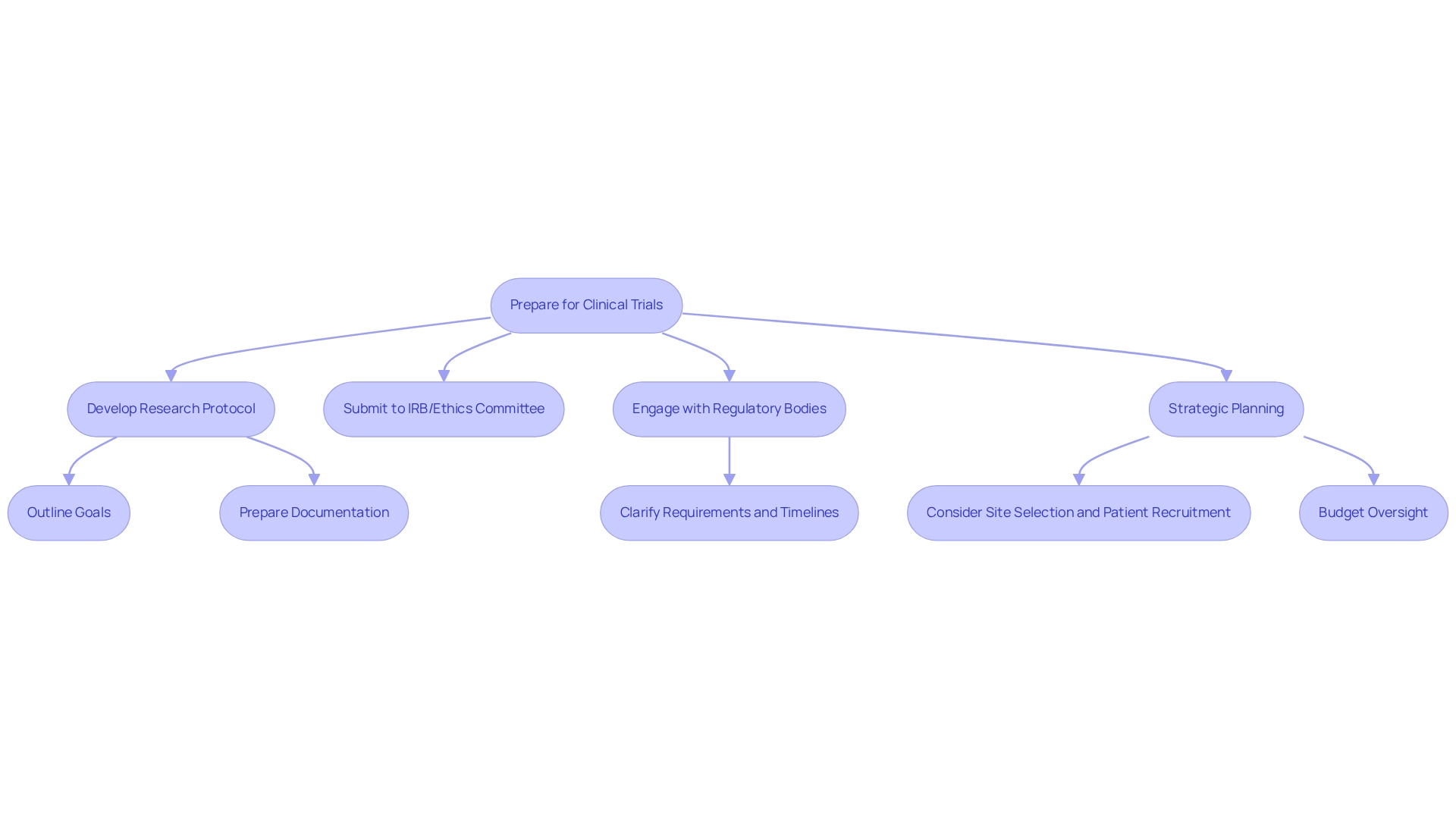
Being prepared for clinical trial innovation in medtech in Chile necessitates a thorough understanding of regulatory adherence and strategic planning. Begin by developing a comprehensive research protocol that explicitly outlines the study's goals, methods, and ethical aspects. This protocol must be submitted to an Institutional Review Board (IRB) or Ethics Committee for approval, ensuring that all required documentation, including proof of ethical approval and risk assessments, is meticulously prepared—an essential step for clinical trial innovation in medtech in Chile. Engaging with local regulatory bodies early in the process is crucial to clarify requirements and timelines. In 2025, the average duration for research approval in Chile is anticipated to be about six months, significantly influenced by the evolving regulatory environment, which highlights the importance of clinical trial innovation in medtech to ensure alignment with regional health priorities. Notably, only 16% of research studies registered between 2010 and 2019 addressed the ten most prevalent reasons for disability-adjusted life years (DALYs) in Chile, revealing a critical gap that clinical trial innovation must address.
Strategic planning is equally vital; consider aspects such as site selection, patient recruitment methods, and budget oversight. Collaborating with local experts or a contract research organization (CRO) like bioaccess® can substantially enhance your planning efforts and ensure compliance with local regulations. bioaccess® provides extensive study management services, including:
- Feasibility Studies
- Site Selection
- Compliance Reviews
- Setup
- Import Permits
- Project Management
- Reporting
These services are essential for the successful execution of studies. As specialists have indicated, clinical trial innovation in medtech in Chile can be significantly improved by regulations and guidelines that foster cooperative relationships between external sponsors and local authorities, alongside increased funding for local researchers, enhancing the adaptability of studies to local health needs. This approach not only aligns with ethical considerations but also ensures that clinical trial innovation is structured to address the specific health challenges faced by the population. Furthermore, continuous assessment of research practices is critical to ensure alignment with local health priorities, emphasizing the necessity for flexible strategies in clinical trial innovation for medtech in Chile.
Execute Clinical Trials: Best Practices for Management and Oversight
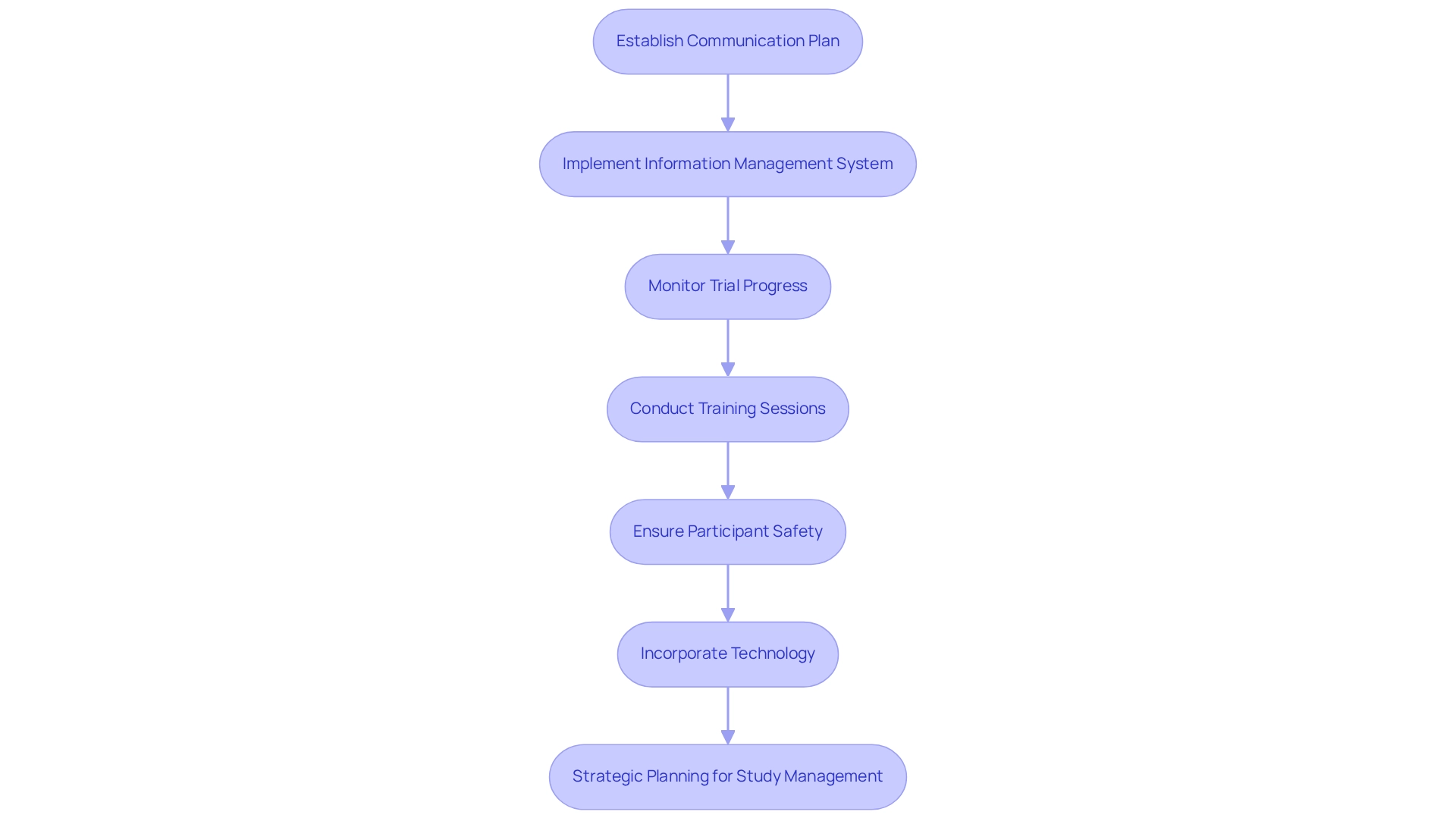
Conducting research studies necessitates meticulous administration and supervision, particularly within the dynamic landscape of Latin America. To begin, establish a comprehensive communication plan that engages all stakeholders, including investigators, sponsors, and regulatory bodies. With bioaccess®'s tailored approach and expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, you can leverage a team with over 20 years of experience in Medtech to effectively navigate these complexities. A robust information management system is essential for ensuring precise and timely information gathering, a factor that is increasingly critical as the sector anticipates significant changes in design processes driven by enhanced collaboration and efficiency in 2025. Insights from IBISWorld underscore the importance of clinical trial information management for optimizing these systems.
Regular monitoring of trial progress against established timelines and budgets is imperative, allowing for necessary adjustments to remain on course. Training sessions for site personnel are vital to ensure familiarity with the research protocol and compliance requirements. Prioritizing participant safety is paramount; adherence to ethical guidelines and transparency with participants regarding their rights and the study's objectives is essential.
Incorporating technology, such as electronic data capture systems, can streamline processes and enhance data accuracy. Notably, in 2023, venture capital funding in research startups reached $4.2 billion, underscoring the sector's commitment to accelerating drug development and enhancing research capabilities. This trend highlights the significance of embracing innovative solutions and optimal methodologies in clinical trial innovation for Medtech in Chile, particularly in Latin America, where navigating the regulatory environment, such as compliance with INVIMA, is crucial for achieving successful market entry. Furthermore, the lack of mid-sized commercial-stage participants presents challenges for financing late-stage studies, emphasizing the need for strategic planning in study management. As Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, noted during his experience with bioaccess® in Colombia, the customized support provided by bioaccess® was instrumental in managing the complexities of their initial human study. This underscores the importance of standardization in trial practices.
Conduct Post-Trial Activities: Data Analysis and Reporting
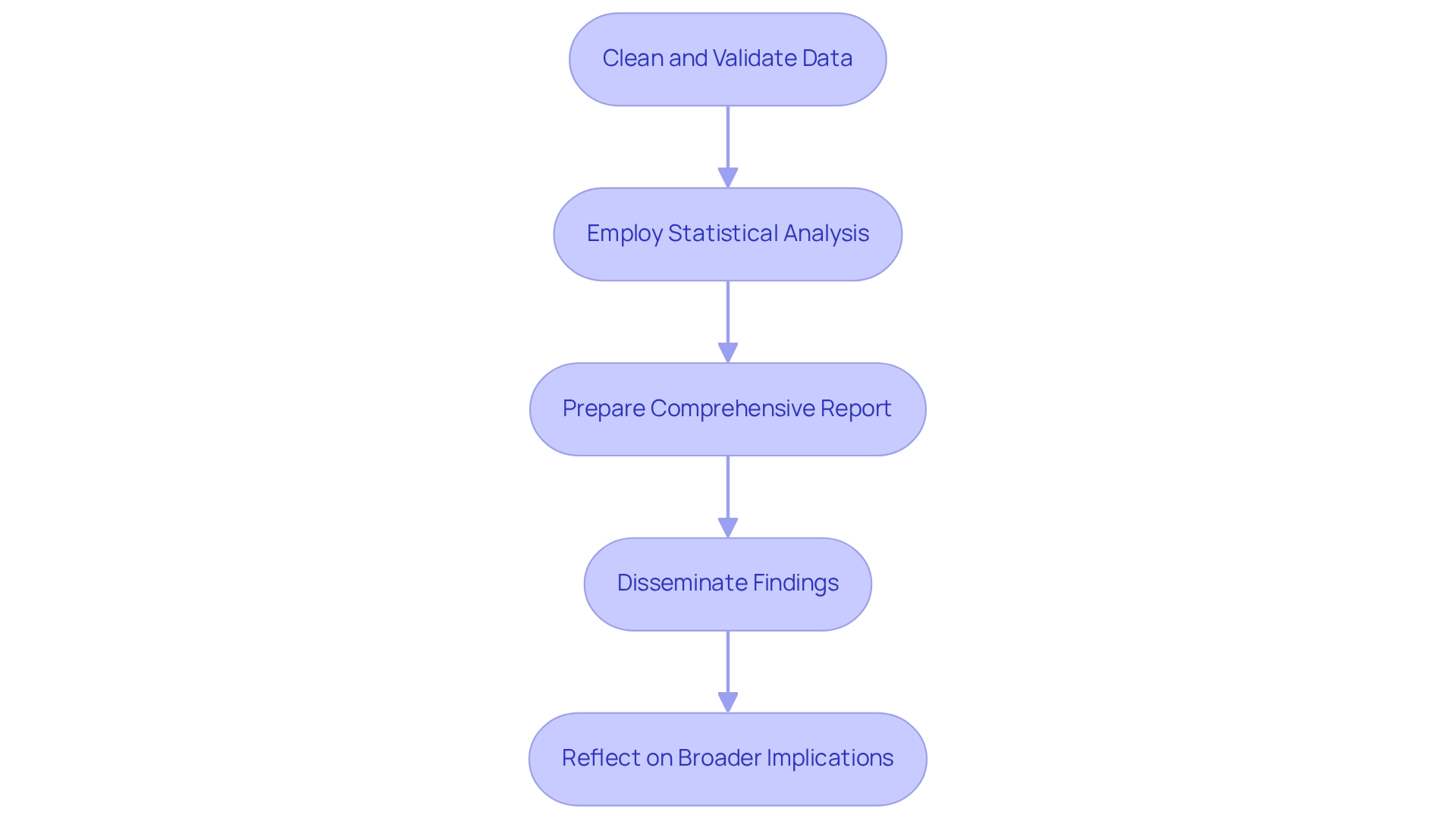
Post-trial activities hinge on a meticulous analysis of information to effectively assess clinical research results. Begin by cleaning and validating the data to uphold its integrity, which is essential for reliable outcomes. Employ statistical analysis techniques tailored to your research design, ensuring a precise interpretation of the findings. A comprehensive report should encapsulate the project’s overview, methodology, results, and conclusions, while also acknowledging any limitations encountered during the experiment.
Disseminating findings is crucial; share your results with stakeholders, including regulatory bodies, healthcare professionals, and the scientific community, through publications and presentations. This transparency not only fosters trust but also enhances the credibility of your research. Additionally, reflect on the broader implications of your findings for future research and practice. Explore options for post-market research or follow-up evaluations to examine the device's effectiveness more thoroughly, thereby aiding the continuous advancement in the Medtech field.
In Chile, the trend where thirty-three out of thirty-six negative studies were either unpublished or misrepresented underscores the pressing need for clinical trial innovation in the medtech sector. This situation highlights the critical importance of precise reporting methods, as specialists assert that the potential for clinical trial innovation in Chile is directly linked to the quality of information gathered and utilized. As Ernest Dimnet aptly stated, "our potential for innovation, problem-solving, and growth is only as good as the information we gather and, most importantly, employ." This reinforces the notion that effective analytics requires not only robust technical tools but also human intuition to pose the right questions, ensuring that the insights obtained are actionable and relevant. By prioritizing best practices in reporting clinical trial outcomes, researchers can significantly enhance the impact of their findings on the Medtech sector, as evidenced by Dr. Jorge Hernando Ulloa's presentation of the one-year first-in-human data for the VenoValve® at the Charing Cross International Symposium, which showcased advancements in vascular medicine.
Conclusion
Chile's emergence as a pivotal player in Medtech clinical trials is underscored by its robust healthcare framework and unwavering commitment to ethical research practices. With a diverse population and an expanding network of healthcare facilities, the country offers fertile ground for innovative clinical studies that can significantly shape healthcare solutions tailored to local needs. Understanding the regulatory landscape, engaging with local expertise, and employing best practices are essential for researchers aiming to navigate the complexities of conducting successful trials in this region.
The significance of strategic planning and regulatory compliance cannot be overstated. By developing detailed protocols, engaging with local regulatory bodies, and leveraging comprehensive clinical trial management services, researchers can markedly enhance their chances of success. The rising trend in trial registrations reflects a steadfast commitment to addressing pressing health challenges, making it imperative for stakeholders to align their efforts with local health priorities.
Moreover, the execution of clinical trials demands diligent management and oversight, emphasizing the critical need for effective communication and participant safety. Incorporating technology and maintaining transparency throughout the process are vital for building trust and ensuring accurate data collection. The post-trial phase is equally crucial, as meticulous data analysis and transparent reporting can profoundly influence future research and innovation in the Medtech sector.
In conclusion, Chile stands out as an optimal location for advancing Medtech innovations through clinical trials, propelled by a supportive regulatory environment, a diverse patient population, and a commitment to ethical standards. By embracing these opportunities and addressing local health challenges, stakeholders can contribute to meaningful advancements in healthcare that resonate with the needs of the Chilean population.
Frequently Asked Questions
What is the significance of Chile in medical research?
Chile has established itself as a leading center for medical research in Latin America, supported by a robust healthcare infrastructure and a high level of medical expertise.
What role does the Instituto de Salud Pública (ISP) play in medical research in Chile?
The ISP oversees an efficient regulatory process that ensures compliance with international standards and promotes ethical research practices, enhancing public confidence in the research process.
What types of examinations are commonly conducted in Chilean medical research?
Common examinations include first-in-human experiments and critical evaluations.
How does Chile's diverse population benefit medical research?
Chile's population of approximately 20 million, which includes various ethnicities and socioeconomic backgrounds, provides a representative sample that enhances the applicability of research findings.
Can you provide an example of a successful study conducted in Chile?
The NeuroStim experiment demonstrated that 70% of participants experienced significant reductions in depression symptoms, showcasing the potential impact of well-structured research within the diverse Chilean demographic.
What recent trends have been observed in study registrations in Chile?
There has been a notable increase in study registrations, with a reported 25% rise in 2025 compared to the previous year, indicating a growing investment in the Medtech sector.
What services does bioaccess® provide to researchers in Chile?
Bioaccess® offers comprehensive study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting on adverse events.
Why is Chile considered an ideal location for advancing Medtech innovations?
Chile's supportive regulatory environment, diverse patient population, commitment to ethical standards, and the ability to address local health needs position it as an ideal location for advancing Medtech innovations.




