Introduction
As the landscape of medical technology continues to evolve, Chile has emerged as a promising destination for Medtech clinical trials. With its stable political climate, advanced healthcare infrastructure, and diverse patient population, the country offers a unique environment conducive to innovative research.
However, navigating the complexities of clinical trials in this region presents its own set of challenges, from regulatory compliance to effective patient recruitment.
This article delves into the myriad advantages of conducting Medtech trials in Chile, the essential services provided by Contract Research Organizations (CROs), and the competitive landscape that shapes this burgeoning hub for clinical research. By exploring these facets, stakeholders can better understand how to leverage Chile's strengths to facilitate successful Medtech innovations.
Chile: An Emerging Hub for Medtech Clinical Trials
Chile has swiftly established itself as an important participant in Medtech CRO Services Chile within the realm of medical technology trials. The nation's stable political atmosphere, combined with a strong healthcare system and an increasing population, makes it an appealing location for conducting medical research, especially for Medtech CRO Services Chile. However, medical technology firms face challenges such as regulatory hurdles, language barriers, and fragmented resources in the region.
The increasing investment in healthcare infrastructure and technology has fostered an ecosystem conducive to innovation. As medical technology firms strive to enhance their research abilities, the Medtech CRO Services Chile offers access to various patient groups, which is vital for the success of research studies. Collaborations such as those between Greenlight Guru and bioaccess™ are pivotal in overcoming these challenges by streamlining processes and enhancing the quality of clinical trials.
Bioaccess®, as a prominent CRO, provides Medtech CRO Services Chile that are crucial for regulatory approval and site activation, thus contributing to accelerated innovations and enhanced patient outcomes in the medical technology sector.

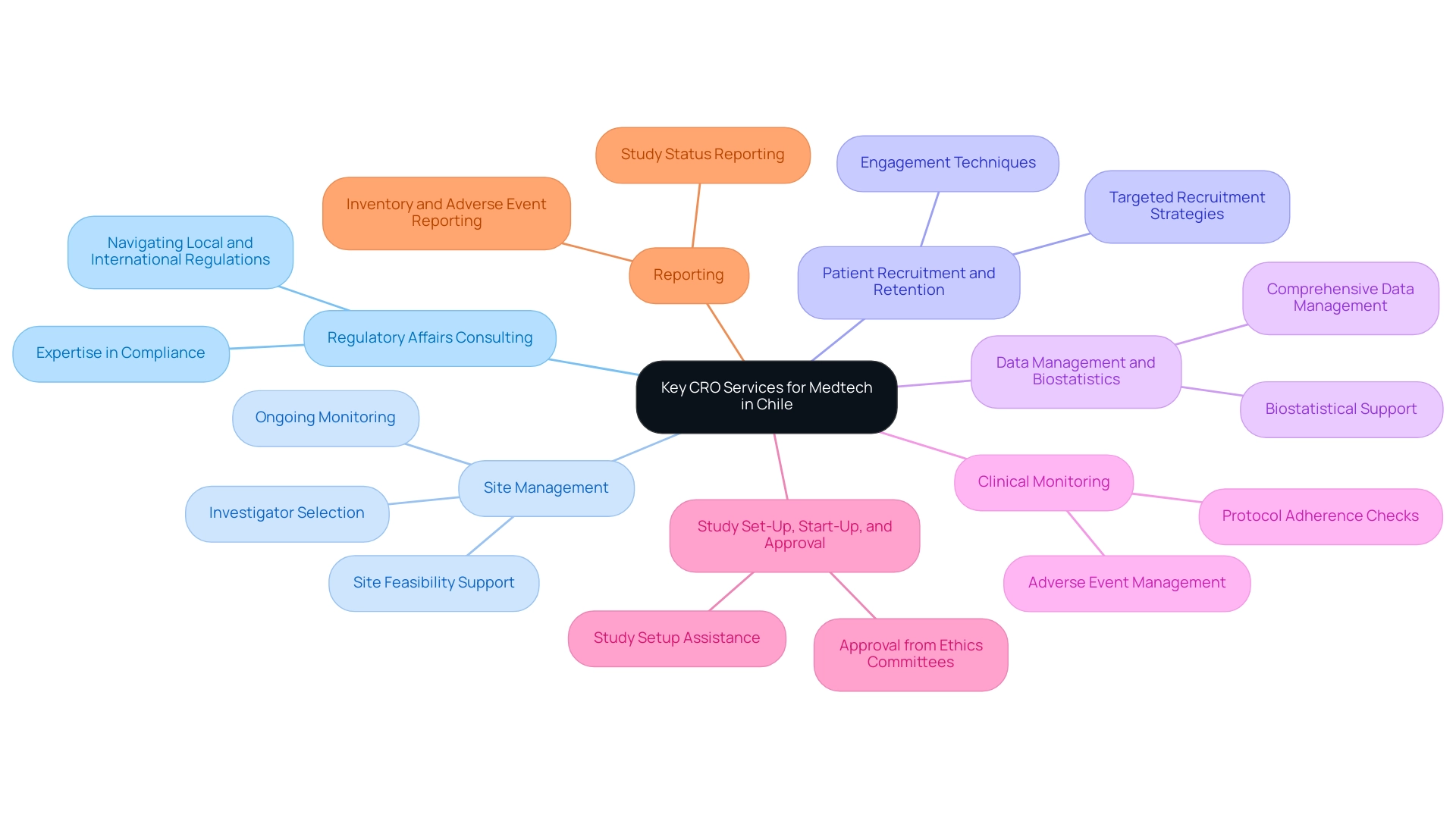
Key CRO Services to Consider for Medtech in Chile
When considering Medtech CRO Services Chile, Medtech companies should look for the following key services:
- Regulatory Affairs Consulting: Navigating the complex regulatory landscape is critical, and CROss like bioaccess® offer extensive expertise in compliance with both local and international regulations, ensuring that medical device studies meet all necessary requirements.
- Site Management: Efficient site oversight is crucial for the seamless implementation of research studies. CROss provide thorough support in site feasibility, investigator selection, and ongoing monitoring as part of their Medtech CRO Services Chile to maintain operational efficiency.
- Patient Recruitment and Retention: Given the challenges in quickly enrolling patients for research studies, Medtech CRO Services Chile implement targeted strategies to attract diverse patient populations and keep participants engaged throughout the study, thereby enhancing the validity of results.
- Data Management and Biostatistics: Robust data management practices are vital for accurate analysis. CROs can offer comprehensive biostatistical support through Medtech CRO Services Chile to effectively interpret clinical data and ensure precise reporting of study outcomes.
- Clinical Monitoring: Regular observation of research sites is crucial to ensure adherence to protocols and quality standards, safeguarding the integrity of the study and addressing any serious or non-serious adverse events efficiently.
- Study Set-Up, Start-Up, and Approval: Medtech CRO Services Chile assist in the critical processes of study set-up, start-up, and obtaining necessary approvals from ethics committees and health ministries.
- Reporting: Comprehensive reporting on study status, inventory, and both serious and non-serious adverse events is provided to maintain transparency and compliance throughout the process.
With more than 20 years of experience in medical technology, the bioaccess® team offers specialized expertise and a tailored strategy to guarantee the success of your research studies.
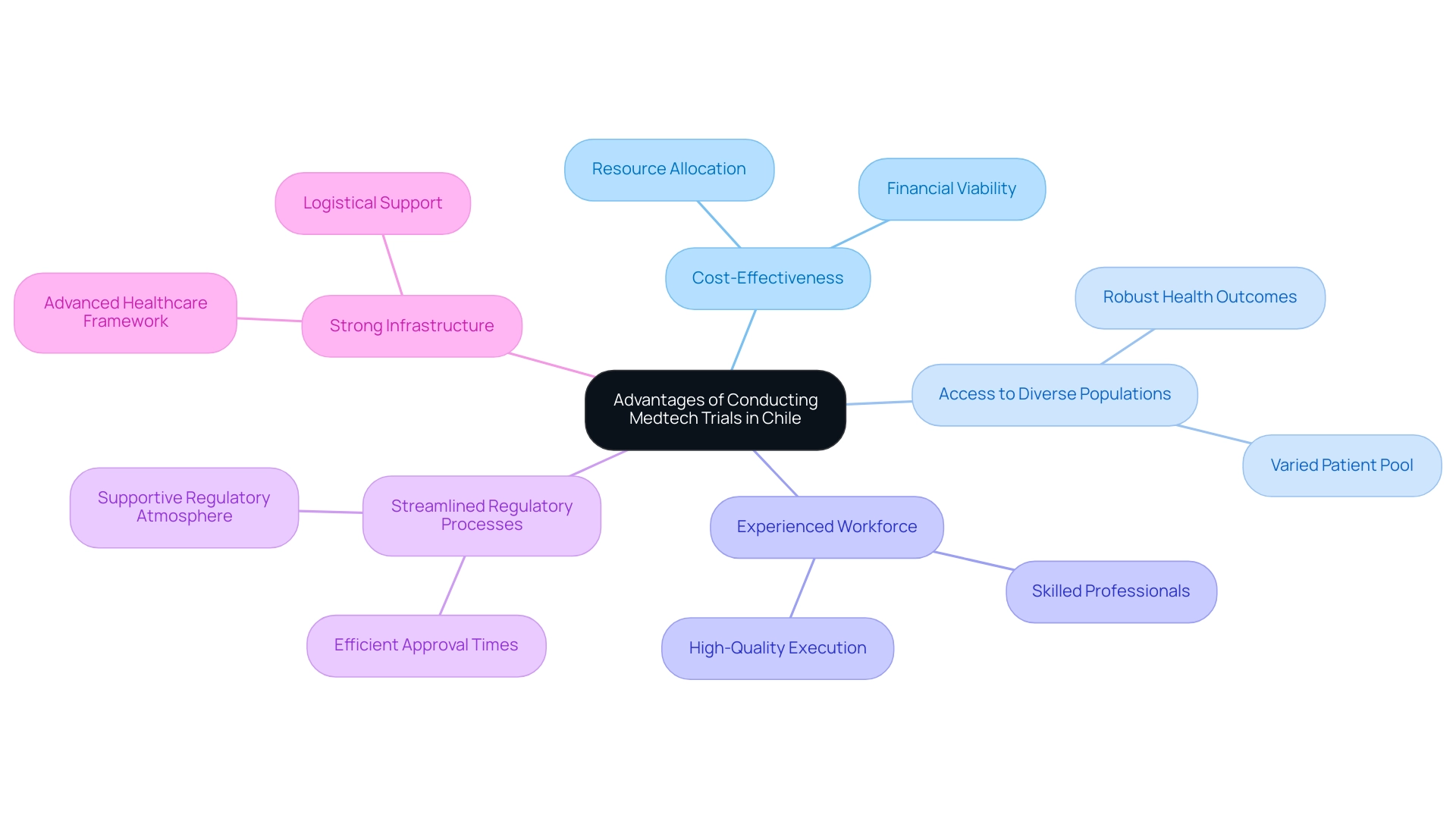
Advantages of Conducting Medtech Trials in Chile
Conducting Medtech CRO Services Chile in the South American nation offers numerous benefits that render it an attractive location for clinical research. These benefits include:
- Cost-Effectiveness: Clinical trials in this region often prove to be more financially viable than those in North America and Europe, enabling companies to allocate their resources more effectively. This cost advantage is crucial for startups and established firms alike, as it allows for more extensive testing and development.
- Access to Diverse Populations: The rich demographic diversity of the country provides a varied patient pool that is essential for studies requiring a wide range of participant profiles. This diversity enhances the robustness of health outcomes and supports the generalizability of research findings. Julio G. Martinez-Clark, a prominent authority in LATAM Medtech CROss, highlights the significance of acknowledging the potential in Latin America, stating, "This country, similar to Colombia, possesses untapped demographics that can greatly aid medical research."
- Experienced Workforce: The country boasts a highly skilled workforce with substantial expertise in medical research. The presence of trained professionals ensures high-quality execution of studies, which is critical for maintaining research integrity and meeting international standards.
- Streamlined Regulatory Processes: While Argentina is recognized for its efficient regulatory procedures, the country to the south also provides a supportive regulatory atmosphere that aids in medical research. This efficiency reduces approval times compared to other regions, vital for companies looking to expedite their development timelines and bring innovations to market faster.
- Strong Infrastructure: Chile's advanced healthcare framework supports the logistical requirements of clinical studies, encompassing everything from patient care to data collection. This infrastructure enhances the overall efficiency of studies and contributes to successful trial outcomes.
Additionally, bioaccess® specializes in managing a range of studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), leveraging over 20 years of experience in Medtech CRO Services Chile. The effect of medical research in Chile extends beyond individual studies. As emphasized in recent talks at the Nordic Health Summit Japan, around 80% of attendees expressed they were likely or very likely to find new business opportunities, highlighting the increasing significance of Latin America as a crucial center for research.
The function of bioaccess® in assisting medical technology startups illustrates the area's dedication to promoting innovation and managing the intricacies of research in healthcare. Collectively, these elements establish the country as a top location for carrying out medical technology studies in 2024 and the future.
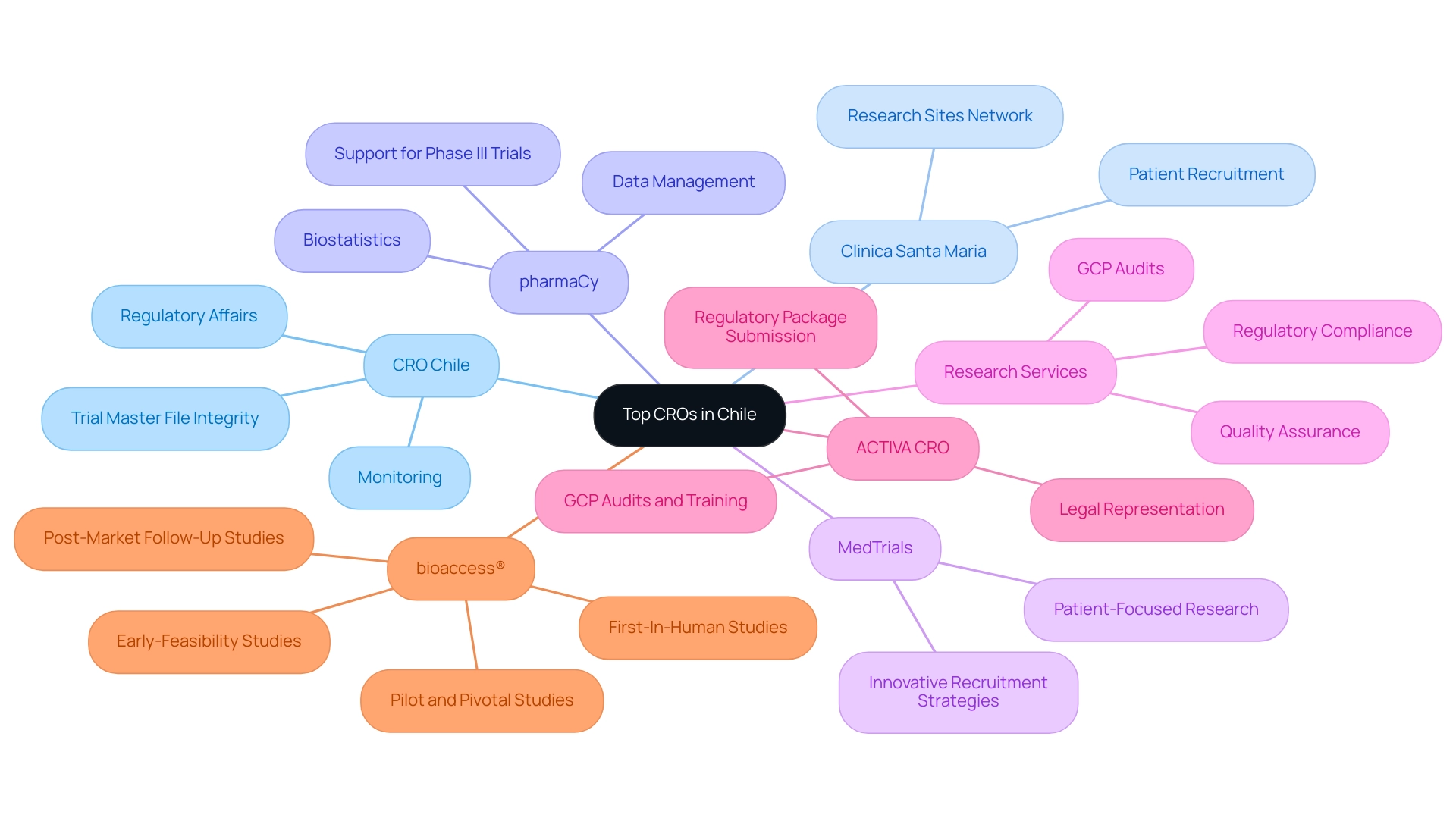
Top CROs in Chile: A Competitive Overview
In the competitive environment of medical trials in South America, several Contract Research Organizations (CROs) distinguish themselves by offering specialized services tailored to the needs of the Medtech industry:
- CRO Chile: Acknowledged for its strong services in regulatory affairs and monitoring, CRO Chile has established its reputation among global sponsors, especially in preserving the integrity of the Trial Master File and ensuring adherence to regulatory standards. Their dedication to source data validation through Medtech CRO Services Chile is crucial for the success of research trials.
- Clinica Santa Maria: This organization excels in patient recruitment, leveraging a vast network of research sites across the country to ensure a diverse participant pool. Their strategic approach enhances the efficiency and effectiveness of research trials.
- pharmaCy: With a specialization in biostatistics and data management, pharmaCy plays a critical role in supporting data-driven decision-making. Their expertise is vital for Phase III trials, where large populations of 500-5,000 or more patients are tested, ensuring that data integrity is maintained throughout the process.
- MedTrials: Recognized for its patient-focused research methodologies, MedTrials utilizes innovative recruitment strategies that have produced successful outcomes across various therapeutic areas, showcasing their dedication to quality and efficiency in trials.
- Research Services: This CRO prioritizes quality assurance and regulatory compliance, making it an invaluable partner for Medtech CRO Services Chile. Their comprehensive Medtech CRO Services Chile encompass performing GCP audits and creating quality plans, ensuring that sponsors fulfill the essential regulatory requirements.
Additionally, ACTIVA CRO has emerged as a significant player by providing legal representation for sponsors seeking to navigate the Latin American research landscape. Their capability to submit regulatory packages and sign contracts through a Power of Attorney allows for seamless project execution. ACTIVA CRO collaborates with sponsors by providing services such as conducting quality GCP audits, internal/external GCP training, and developing Quality Plans and Manuals.
Furthermore, bioaccess® exemplifies the expertise needed to drive successful research studies, focusing specifically on Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). Their comprehensive service capabilities include feasibility and selection of research locations, compliance reviews, study setup, import permits, project management, and detailed reporting. The impact of these medical technology clinical studies extends beyond trial success; they create jobs, promote economic growth, and improve healthcare, ultimately contributing to international collaboration and recognition in the region.
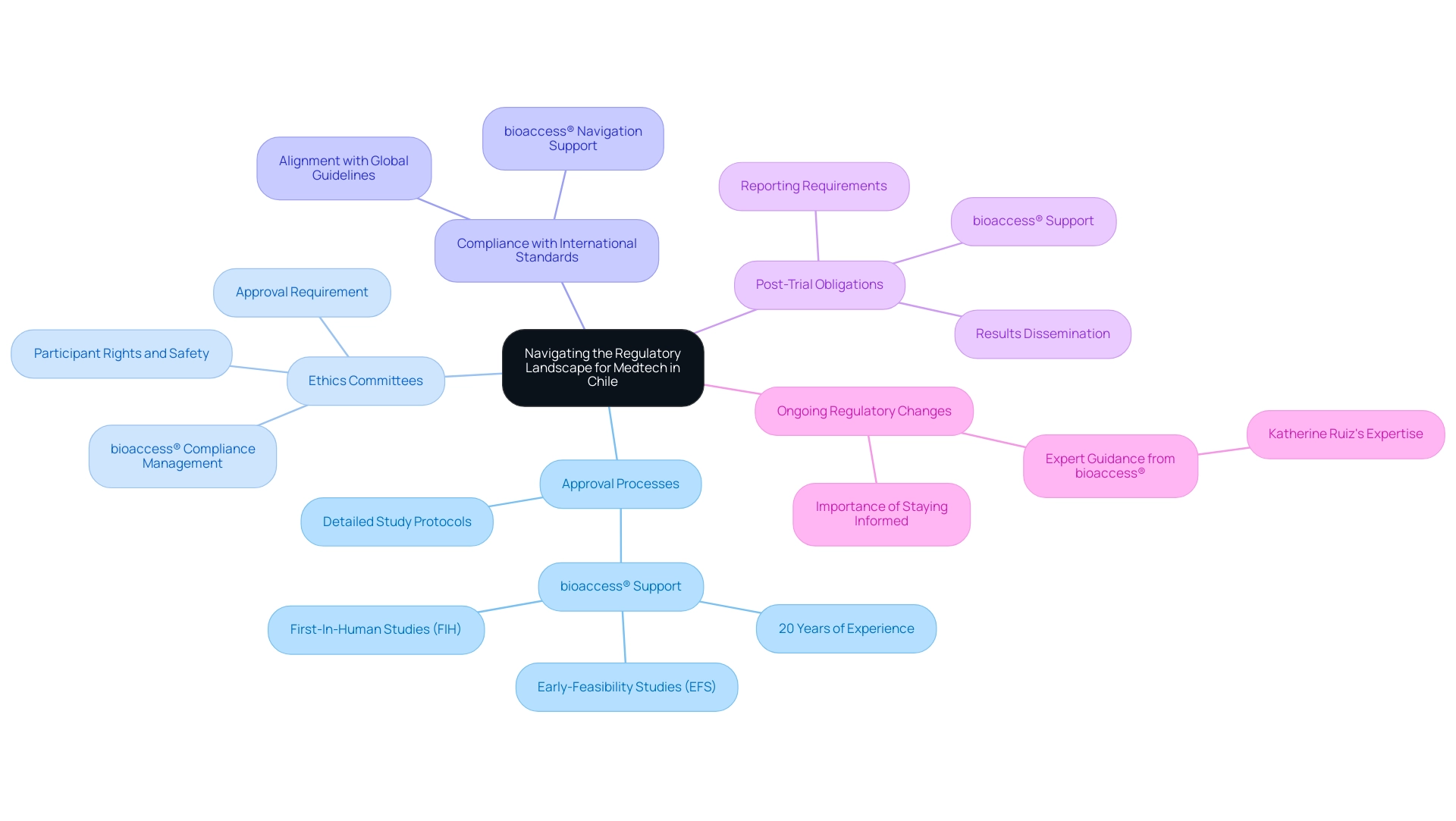
Navigating the Regulatory Landscape for Medtech in Chile
Navigating the regulatory landscape in Chile involves understanding several key elements:
-
Approval Processes:
Medtech companies must submit detailed study protocols to the Chilean regulatory authority, which reviews applications for ethical and scientific validity. Collaborating with a seasoned organization such as bioaccess®, which provides Medtech CRO Services Chile, can simplify this process, as they have more than 20 years of knowledge in overseeing research studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
-
Ethics Committees:
All research studies require approval from local ethics committees, ensuring that participant rights and safety are prioritized. bioaccess® ensures compliance through meticulous oversight and management of these approvals in accordance with Medtech CRO Services Chile.
-
Compliance with International Standards:
Chilean regulations align with international guidelines, providing a familiar framework for global sponsors. Bioaccess® adeptly navigates these standards, ensuring that all study protocols for Medtech CRO Services Chile meet requisite compliance.
-
Post-Trial Obligations:
Companies must adhere to post-trial reporting requirements, including the dissemination of results to participants and stakeholders. bioaccess® supports clients in fulfilling these obligations effectively with Medtech CRO Services Chile.
-
Ongoing Regulatory Changes:
Staying informed about regulatory changes is crucial, as the landscape can evolve, affecting operations and compliance. With experts like Katherine Ruiz, a Regulatory Affairs specialist with extensive experience at INVIMA, bioaccess® is well-equipped to offer Medtech CRO Services Chile, guiding companies through these changes to ensure continuous compliance and successful outcomes.
Additionally, bioaccess® prides itself on its flexibility in managing clinical trials as part of its Medtech CRO Services Chile, adapting to the unique needs of each study to ensure optimal results.
Conclusion
Chile stands out as an emerging hub for Medtech clinical trials, thanks to its combination of a stable political climate, robust healthcare infrastructure, and access to diverse patient populations. These advantages make it an attractive choice for Medtech companies looking to conduct clinical research. However, navigating the complexities of the regulatory landscape and patient recruitment presents challenges that must be addressed through strategic collaboration with experienced Contract Research Organizations (CROs).
CROs in Chile, such as bioaccess®, play a pivotal role in ensuring the success of clinical trials by providing essential services, including:
- Regulatory affairs consulting
- Site management
- Patient recruitment
- Data management
These organizations not only streamline the clinical trial process but also enhance the quality of research outcomes, ultimately contributing to the rapid advancement of Medtech innovations.
The competitive landscape of CROs in Chile further emphasizes the importance of selecting the right partner to navigate the intricacies of clinical trials. By leveraging Chile's strengths—cost-effectiveness, experienced workforce, and streamlined regulatory processes—Medtech companies can facilitate successful research endeavors. As the Medtech industry continues to evolve, Chile's position as a leading destination for clinical trials is set to grow, fostering innovation that will benefit both the local and global healthcare landscape.
Frequently Asked Questions
What is the significance of Chile in Medtech CRO Services?
Chile has become an important player in Medtech CRO Services due to its stable political environment, strong healthcare system, and growing population, making it an attractive location for medical research.
What challenges do medical technology firms face in Chile?
Medical technology firms in Chile encounter challenges such as regulatory hurdles, language barriers, and fragmented resources.
How does investment in healthcare infrastructure affect Medtech CRO Services in Chile?
Increased investment in healthcare infrastructure and technology has created an ecosystem that fosters innovation, allowing Medtech CRO Services in Chile to access diverse patient groups essential for successful research studies.
What key services should Medtech companies look for in Medtech CRO Services Chile?
Key services include Regulatory Affairs Consulting, Site Management, Patient Recruitment and Retention, Data Management and Biostatistics, Clinical Monitoring, Study Set-Up, Start-Up and Approval, and Reporting.
What are the benefits of conducting clinical trials in Chile?
Benefits include cost-effectiveness, access to diverse populations, an experienced workforce, streamlined regulatory processes, and a strong healthcare infrastructure.
What role does bioaccess® play in Medtech CRO Services Chile?
Bioaccess® provides crucial services for regulatory approval, site activation, and managing various studies, leveraging over 20 years of experience to enhance patient outcomes and accelerate innovation.
How does the regulatory landscape in Chile affect Medtech companies?
Companies must navigate approval processes, ethics committee requirements, compliance with international standards, post-trial obligations, and ongoing regulatory changes, often with the support of experienced CROs like bioaccess®.
What is the importance of patient recruitment in Medtech studies?
Effective patient recruitment is essential to ensure diverse participant profiles and maintain engagement throughout studies, which enhances the validity of research results.
How do Chilean regulations align with international standards?
Chilean regulations are designed to align with international guidelines, providing a familiar framework for global sponsors to navigate.
What specific studies does bioaccess® focus on?
Bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).

