Introduction
The landscape of medtech clinical trials in Latin America is rapidly transforming, positioning the region as an attractive destination for innovative research and development. With its diverse patient populations and favorable regulatory environments, countries like Brazil, Mexico, and Argentina are drawing the attention of U.S. medtech startups seeking to navigate the complexities of clinical trials. However, these opportunities are accompanied by significant challenges, including regulatory hurdles and communication barriers that can complicate collaborations with local healthcare institutions.
As stakeholders explore this evolving terrain, understanding the key factors in selecting the right Contract Research Organization (CRO) becomes crucial. This article delves into the essential criteria for evaluating CRO capabilities, emphasizing the importance of experience, regulatory knowledge, and patient engagement strategies in ensuring successful clinical trial outcomes in this burgeoning market.
The Evolving Landscape of Medtech Clinical Trials in Latin America
Latin America has become a significant center for medtech studies because of its varied patient groups, flexible guidelines, and affordability. However, U.S. Medtech startups encounter significant challenges in this region, including:
- Compliance hurdles
- Language barriers
- Fragmented resources
These challenges can impede seamless collaboration with local hospitals. Nations like Brazil, Mexico, and Argentina are becoming more appealing for medical research, motivated by supportive oversight conditions and an increasing number of research sites. The area's regulatory bodies are actively working to simplify approval procedures, making it easier for sponsors to begin and carry out studies.
Moreover, the partnership between Greenlight Guru and bioaccess™ illustrates the dedication to speeding up medtech advancements and studies in Latin America, emphasized by the successful first-in-human research carried out by PAVmed in Colombia. Bioaccess® addresses the challenges faced by U.S. Medtech companies by facilitating communication and collaboration with Latin American hospitals, positioning itself as a leading CRO in the region.
Understanding these dynamics is essential for stakeholders aiming to select the right CRO that can navigate this evolving landscape effectively, leveraging the untapped potential of Latin America for Medtech startups.
Key Criteria for Selecting an Experienced Medtech CRO
Selecting a medtech CRO in Latin America involves careful consideration of several crucial criteria:
- Experience and Track Record: Prioritize a CRO with a proven history of successfully managing medtech clinical studies. For instance, bioaccess® has over 20 years of expertise in conducting various studies, such as Early-Feasibility Studies (EFS) and First-In-Human (FIH) trials, which are increasingly prevalent in countries like Colombia and Paraguay. Scrutinize their portfolio to evaluate their experience with products similar to yours and within relevant therapeutic areas.
- Knowledge of Regulations: It is imperative that the CRO possesses an in-depth understanding of the local compliance landscape, including the ability to navigate the approval processes efficiently. This is particularly important in Brazil and Mexico, where familiarity with requirements from governing bodies such as ANVISA and COFEPRIS can streamline your project timeline. Adrian Ebner, a globally recognized cardiovascular surgeon, highlights the importance of compliance knowledge, asserting that it is essential for the successful implementation of clinical studies, especially within the intricate systems of these nations. Additionally, understanding INVIMA, Colombia's National Food and Drug Surveillance Institute, is vital for compliance and oversight in medical device matters. Katherine Ruiz, a regulatory affairs expert with extensive experience at INVIMA, brings valuable insights into navigating these regulatory challenges, having previously facilitated import licenses for medical devices and diagnostic products.
- Quality Assurance Processes: Investigate the CRO's quality management systems and their adherence to Good Clinical Practice (GCP). A robust emphasis on quality assurance is essential for maintaining compliance and ensuring the integrity of research data, which is fundamental in building trust with stakeholders.
- Site Management and Patient Recruitment: Evaluate the CRO's abilities in overseeing sites and their approaches for recruiting participants. Their capacity to reach diverse patient groups can significantly impact study timelines and results, especially in an area as varied as Latin America, which offers a rich demographic landscape for research.
- Communication and Collaboration: A successful partnership hinges on clear communication and effective collaboration. Assess the CRO's responsiveness and their dedication to interacting with your team during the process. Ensuring alignment on goals and expectations can lead to more efficient project execution. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, and Dr. John B. Simpson, CEO at Avinger, have both emphasized their favorable experiences with bioaccess® during their research activities in Colombia, reflecting the significance of robust collaborative partnerships.
By focusing on these criteria, stakeholders can select a CRO that not only meets their technical needs but also aligns strategically with their research goals. The potential for growth in Latin America's research capabilities is substantial, with the region currently producing only 3% of the world’s research, underscoring the need for strategic investments in human capital and infrastructure to bridge the gap with other areas. Furthermore, the attractiveness of Latin American CROs to international manufacturers highlights the relevance of these criteria in today's competitive landscape. bioaccess® provides a comprehensive range of services, including feasibility studies, site selection, setup, import permits, project management, and reporting, which are essential for successful research studies.
Evaluating the CRO’s Capabilities and Resources
When evaluating a CRO's capabilities, consider the following aspects:
- Technological Infrastructure: Evaluate if the CRO employs advanced technologies for data management, patient monitoring, and study management. Tools such as electronic data capture (EDC) systems and study management systems (CTMS) can improve data precision and simplify processes.
- Staff Expertise: Review the qualifications and experience of the CRO’s team. At bioaccess®, our experts offer more than 20 years of experience in managing medical device research studies, ensuring specialized knowledge in various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, and Post-Market Follow-Up Studies (PMCF). Our team’s flexibility allows us to adapt to the unique challenges of each trial, ensuring tailored solutions for our clients.
- Site Network and Partnerships: Investigate the CRO’s network of clinical sites and relationships with key opinion leaders (KOLs) in the medtech field. A strong site network can facilitate patient recruitment and improve study timelines, which we prioritize at bioaccess®.
- Oversight Support: Ensure the CRO has dedicated affairs specialists, like Katherine Ruiz, who assist with submissions and interactions with oversight authorities, including INVIMA, Colombia's National Food and Drug Surveillance Institute. Katherine's extensive experience at INVIMA equips her to navigate regulatory challenges effectively, ensuring compliance throughout the process and enhancing the likelihood of successful outcomes.
By thoroughly assessing these capabilities, stakeholders can ensure that the CRO they select is well-equipped to handle the complexities of their clinical studies.
Understanding the CRO’s Approach to Patient Engagement
To understand a CRO's approach to patient engagement, consider the following factors:
-
Recruitment Strategies: Inquire about the methods the CRO uses to recruit participants. Effective strategies may include community outreach, patient advocacy partnerships, and digital marketing campaigns to reach diverse populations. Bioaccess® leverages its 20+ years of Medtech expertise to implement targeted recruitment strategies tailored for the Latin American market, including feasibility studies to identify suitable populations.
-
Patient Education: Evaluate how the CRO informs potential participants about the study process, risks, and benefits. Bioaccess® emphasizes clear, accessible information to enhance patient understanding and willingness to participate in various study types, including Early-Feasibility and First-In-Human studies. This is complemented by compliance reviews to ensure that all educational materials meet regulatory standards.
-
Retention Programs: Evaluate the CRO’s strategies for retaining participants throughout the study. This may include regular communication, reminders for follow-up visits, and support services to address patient needs. With comprehensive project management, bioaccess® ensures continuous engagement with participants, fostering retention through personalized support, while also monitoring compliance with study protocols.
-
Feedback Mechanisms: Understand how the CRO gathers and integrates patient feedback into the study process. Involving patients in the research can lead to enhancements in study design and execution. Bioaccess® actively seeks participant feedback to refine its study protocols, ensuring a patient-centered approach that is integral to its research setup and management processes.
By prioritizing patient engagement and integrating these strategies into its comprehensive study management services, stakeholders can enhance recruitment and retention, ultimately contributing to the success of their research initiatives, particularly in the context of accelerated medical device studies in Latin America.
Assessing the CRO’s Financial Stability and Transparency
When evaluating a CRO’s financial stability and transparency, especially in the context of comprehensive clinical study management services, consider the following:
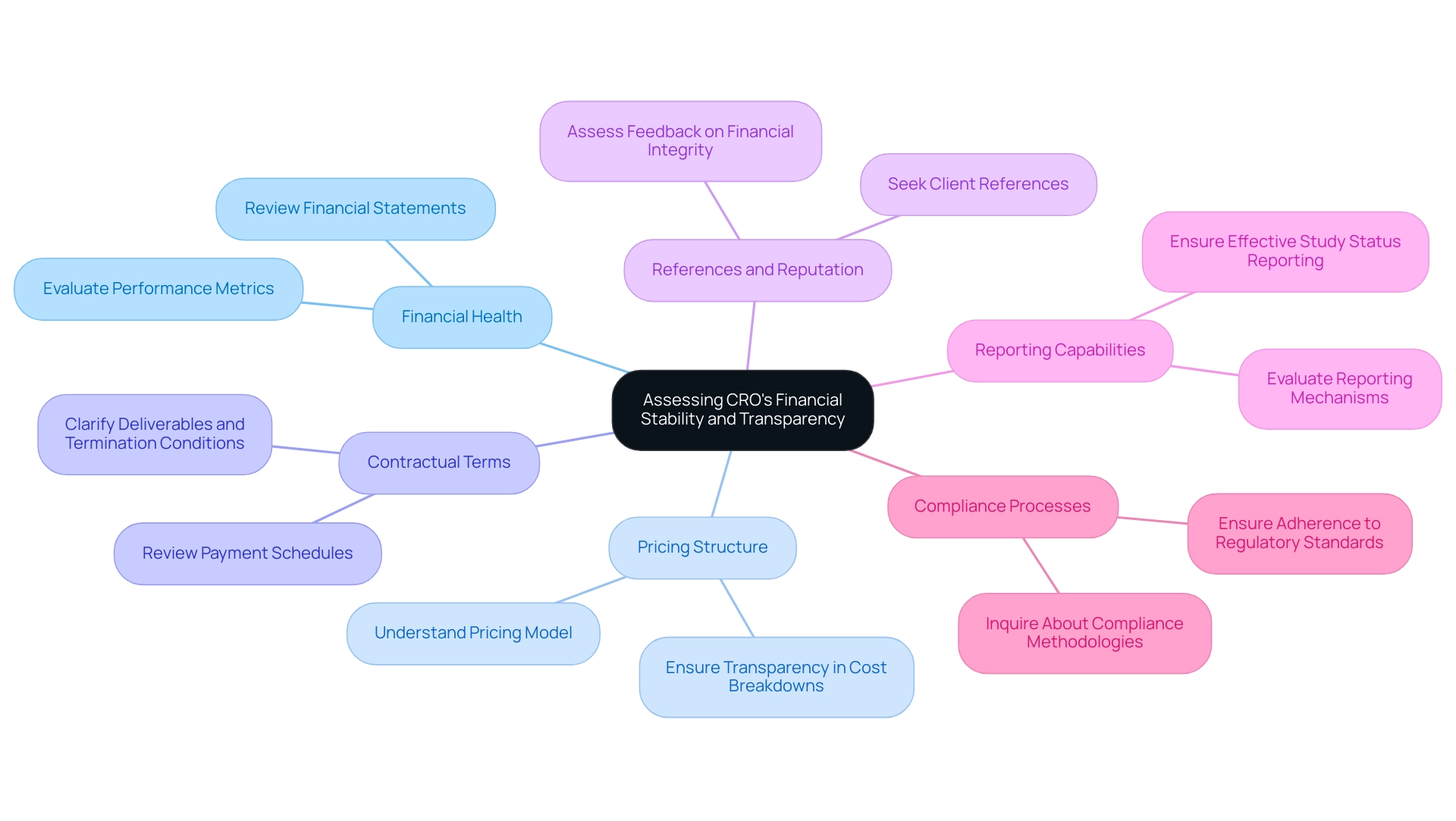
- Financial Health: Review the CRO’s financial statements and performance metrics to gauge their stability. A financially sound CRO, such as bioaccess™, which specializes in feasibility studies, site selection, compliance reviews, and setup, is better positioned to manage unexpected challenges during a study.
- Pricing Structure: Understand the CRO’s pricing model and ensure it aligns with your budget. Look for transparency in cost breakdowns to avoid hidden fees, especially as bioaccess™ provides clear insights into their pricing for services like import permits and project management.
- Contractual Terms: Carefully review the terms of the contract, including payment schedules, deliverables, and conditions for termination. Clear and fair contractual terms can prevent disputes later on. The expertise of a CRO like bioaccess™, vetted and approved to assist U.S. medical device companies in Colombia, can enhance the negotiation process.
- References and Reputation: Seek references from previous clients to gauge the CRO’s reputation in the industry. Positive feedback regarding financial integrity and transparency can provide additional assurance, particularly when the CRO has proven experience in managing complex regulatory and commercialization pathways.
- Reporting Capabilities: Ensure the CRO has robust reporting mechanisms in place. Effective reporting on study status, inventory, and adverse events is crucial for maintaining transparency and accountability throughout the research process. bioaccess™ excels in providing detailed reports that contribute to informed decision-making.
- Compliance Processes: Inquire about how the CRO ensures compliance with country requirements during the trial. bioaccess™ has established methodologies to navigate regulatory landscapes, ensuring all aspects of the study meet necessary standards.
By conducting thorough financial due diligence, stakeholders can select a CRO that not only meets their operational needs but also provides a reliable and stable partnership, evident in bioaccess™’s successful track record.
Conclusion
The transformation of medtech clinical trials in Latin America presents a unique opportunity for U.S. startups looking to expand their reach. With countries like Brazil, Mexico, and Argentina emerging as viable locations, understanding the complexities of the region is essential. Key factors such as the experience of Contract Research Organizations (CROs), their regulatory knowledge, and patient engagement strategies are critical in navigating this evolving landscape.
Choosing the right CRO involves evaluating their track record, quality assurance processes, and communication capabilities. A CRO's ability to manage diverse patient populations and effectively recruit participants can significantly impact trial success. Furthermore, the financial stability and transparency of a CRO are paramount, ensuring that stakeholders can establish reliable partnerships that support their clinical objectives.
As the medtech sector continues to grow in Latin America, strategic investments in research infrastructure and human capital will be necessary to enhance the region's capabilities. By prioritizing the outlined criteria in selecting a CRO, stakeholders can not only mitigate risks but also harness the vast potential that Latin America offers for innovative clinical research. The commitment to fostering strong collaborations and maintaining high standards in clinical trial management will ultimately lead to successful outcomes and advancements in the medtech industry.
Frequently Asked Questions
Why is Latin America considered a significant center for medtech studies?
Latin America is viewed as a significant center for medtech studies due to its varied patient groups, flexible guidelines, and affordability, which attract medical research.
What challenges do U.S. medtech startups face in Latin America?
U.S. medtech startups encounter several challenges in Latin America, including compliance hurdles, language barriers, and fragmented resources, which can hinder collaboration with local hospitals.
Which countries in Latin America are becoming more appealing for medical research?
Countries such as Brazil, Mexico, and Argentina are becoming more appealing for medical research due to supportive oversight conditions and a growing number of research sites.
How are regulatory bodies in Latin America addressing approval procedures for medtech studies?
Regulatory bodies in Latin America are actively working to simplify approval procedures, making it easier for sponsors to initiate and conduct studies.
What is the role of bioaccess® in facilitating medtech advancements in Latin America?
Bioaccess® aids U.S. medtech companies by enhancing communication and collaboration with Latin American hospitals, positioning itself as a leading Contract Research Organization (CRO) in the region.
What criteria should stakeholders consider when selecting a medtech CRO in Latin America?
Stakeholders should consider the CRO's experience and track record, knowledge of regulations, quality assurance processes, site management and patient recruitment, and communication and collaboration capabilities.
Why is knowledge of local regulations important for a CRO?
Knowledge of local regulations is crucial for a CRO to navigate approval processes efficiently, particularly in countries like Brazil and Mexico, where compliance with governing bodies can streamline project timelines.
What aspects should be evaluated regarding a CRO's capabilities?
Evaluation should include the CRO's technological infrastructure, staff expertise, site network and partnerships, and oversight support to ensure they can handle the complexities of clinical studies.
How does bioaccess® approach patient engagement in studies?
Bioaccess® employs targeted recruitment strategies, emphasizes patient education, implements retention programs, and gathers participant feedback to enhance engagement and study success.
What factors should be assessed to determine a CRO's financial stability and transparency?
Stakeholders should review the CRO’s financial health, pricing structure, contractual terms, references and reputation, reporting capabilities, and compliance processes to ensure a reliable partnership.

