Introduction
In recent years, Contract Research Organizations (CROs) in Latin America have emerged as indispensable allies in the pharmaceutical and medical device sectors, facilitating the complex landscape of clinical trials. With a staggering 88.24% revenue share in the clinical segment as of 2023, these organizations not only streamline research processes but also leverage local expertise to enhance trial efficiency and patient engagement. As the demand for innovative healthcare solutions grows, the role of CROs becomes increasingly critical, navigating diverse regulatory frameworks while ensuring compliance and ethical standards.
This article delves into the multifaceted contributions of Latin American CROs, examining their impact on clinical research, patient recruitment, and the evolving regulatory environment, ultimately highlighting their pivotal position in advancing medical knowledge and innovation across the region.
Defining Contract Research Organizations (CROs) in Latin America
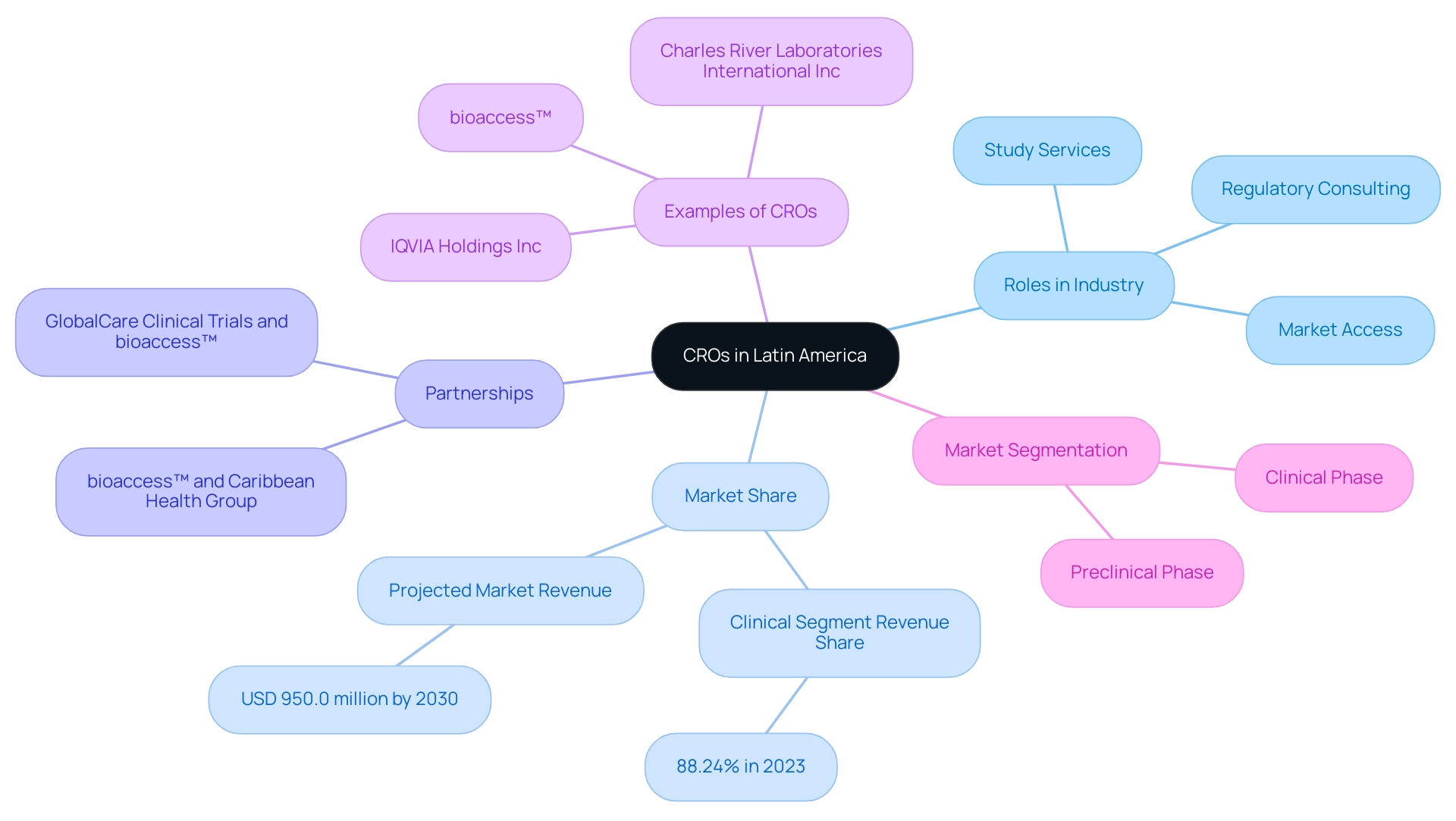
In Latin America, Contract Research Organizations act as vital collaborators for the pharmaceutical, biotechnology, and medical device industries, providing external study services that are crucial to the success of trials. With a significant revenue share of 88.24% in the medical segment in 2023, CROs are crucial in handling the complexities inherent in projects.
As Anil Kumar P., Research Manager - Healthcare, states, "Whether collaborating with small start-ups or large enterprises, Anil consistently delivers high-quality analysis that supports informed decision-making and drives impactful results in the healthcare industry."
These organizations vary significantly in size, from specialized firms targeting specific therapeutic areas to large multinational corporations capable of executing extensive global studies. Significantly, bioaccess™ has positioned itself as a prominent CRO in Latin America, partnering with Caribbean Health Group to establish Barranquilla as a prime location for research, backed by Colombia's Minister of Health. This partnership seeks to improve the region's appeal for medical research, resulting in greater investment and innovation.
Furthermore, GlobalCare Clinical Trials has teamed up with bioaccess™ to improve ambulatory services in Colombia, achieving over a 50% decrease in recruitment time and an impressive 95% retention rate, highlighting the effectiveness of their strategy. By utilizing local knowledge, clinical research organizations can effectively maneuver through the region’s regulatory environments, enlist varied patient groups, and optimize study schedules. This adaptability not only improves the effectiveness of research studies but also aids in the broader progress of medical understanding and innovation in the healthcare sector.
The market segmentation encompasses preclinical and experimental phases, with the experimental segment being the largest, showcasing key players such as IQVIA Holdings Inc and Charles River Laboratories International Inc. As the market is expected to attain USD 950.0 million by 2030, the rising demand for medical device innovation emphasizes the vital role of organizations like bioaccess™ in experimental activities across Latin America.
Furthermore, bioaccess™ plays a critical role in regulatory and market access consulting for companies like Welwaze Medical Inc., ensuring a smooth entry into the Colombian market for innovative medical devices like Celbrea®. This comprehensive support is vital for Medtech startups seeking expedited research study results.
The Role of Latin American CROs in Advancing Clinical Research
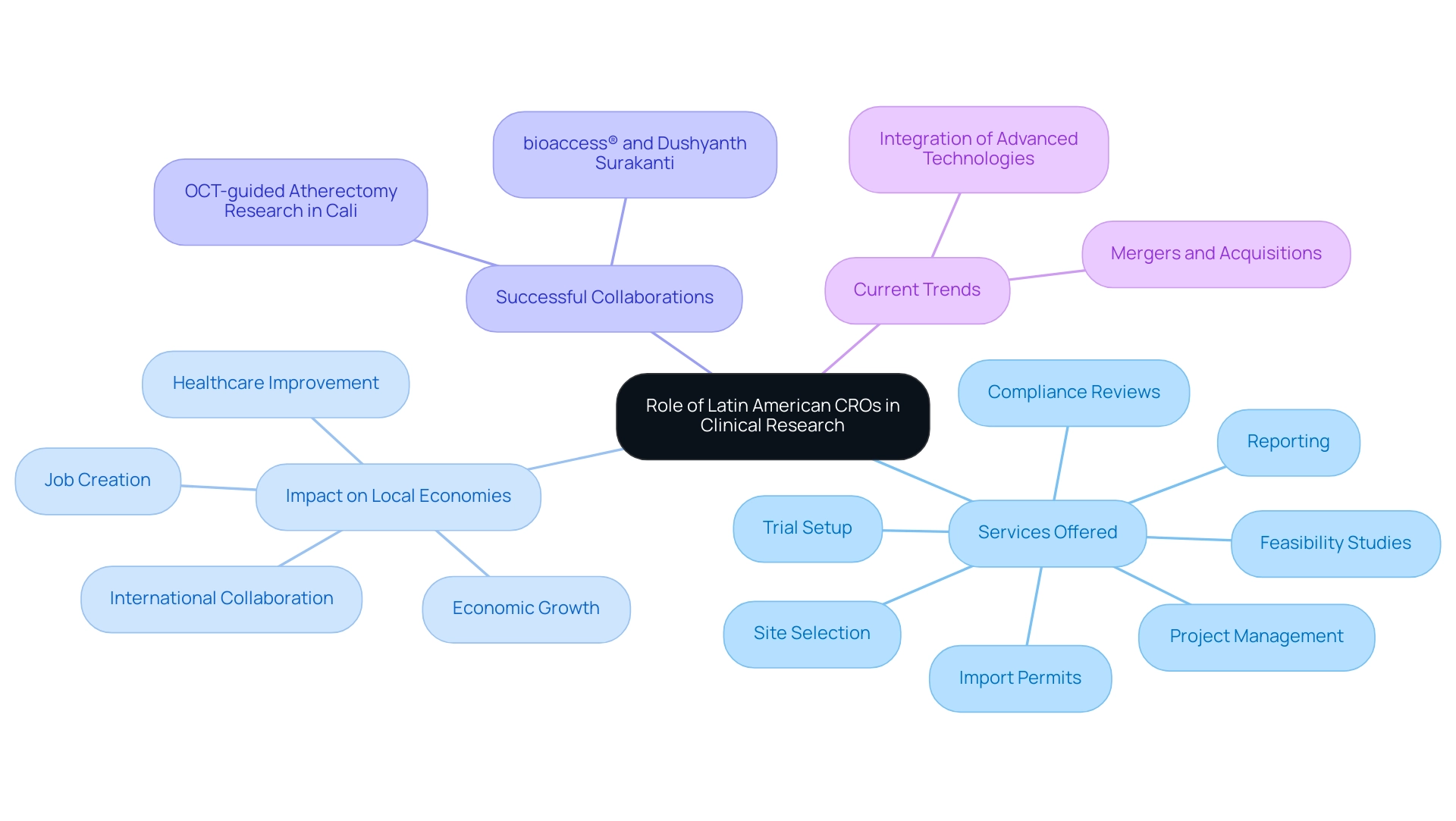
Latin American Contract Research Organizations are essential in promoting medical studies, utilizing their regional expertise and resources to enhance study management. With local pharmaceutical companies reporting outsourcing rates between 50% and 70%, CROs in the region are particularly attractive to sponsors aiming to stretch their research budgets efficiently. These organizations not only offer affordable solutions for study management but also have access to varied patient populations, producing research results that more accurately represent real-world situations.
For example, Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his experience with bioaccess® during its initial human study in Colombia, highlighting the significance of local expertise. Likewise, Dr. John B. Simpson, CEO of Avinger, highlighted the successful implementation of OCT-guided atherectomy research in Cali, Colombia, demonstrating collaboration with LATAM CRO specialists.
The comprehensive services provided by bioaccess®, including:
- feasibility studies
- site selection
- compliance reviews
- trial setup
- import permits
- project management
- reporting
are crucial for the effective execution of research trials. Moreover, the impact of Medtech research studies on local economies is profound, creating jobs, promoting economic growth, improving healthcare, and fostering international collaboration.
Bioaccess® stands as a leader in Medtech clinical research in Latin America, focusing on innovation and regulatory excellence, which further enhances the strategic advantages these organizations provide. Current trends indicate that Latin American contract research organizations are increasingly enhancing their service offerings, including the integration of advanced technologies and methodologies to improve trial efficiency. Additionally, many contract research organizations are considering mergers and acquisitions with local firms to expand their capabilities and better serve the bio-pharmaceutical sector.
Despite obstacles like differing regulatory structures and cultural disparities, the strategic benefits provided by Latin American contract research organizations make them essential allies in the medical field, promoting innovation and improving patient outcomes across the region.
Regulatory Framework and Compliance in Latin America
The regulatory landscape for clinical research in Latin America presents a diverse and intricate challenge, as regulations differ significantly across countries. For instance, Brazil, Mexico, and Argentina each have distinct regulatory frameworks that contract research organizations must navigate.
In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) plays a crucial role in overseeing medical devices and ensuring compliance with health regulations, classified as a Level 4 health authority by PAHO/WHO. Contract Research Organizations (CROs) are tasked with managing this complex array of regulations imposed by national health authorities, ethics committees, and international guidelines. They provide extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
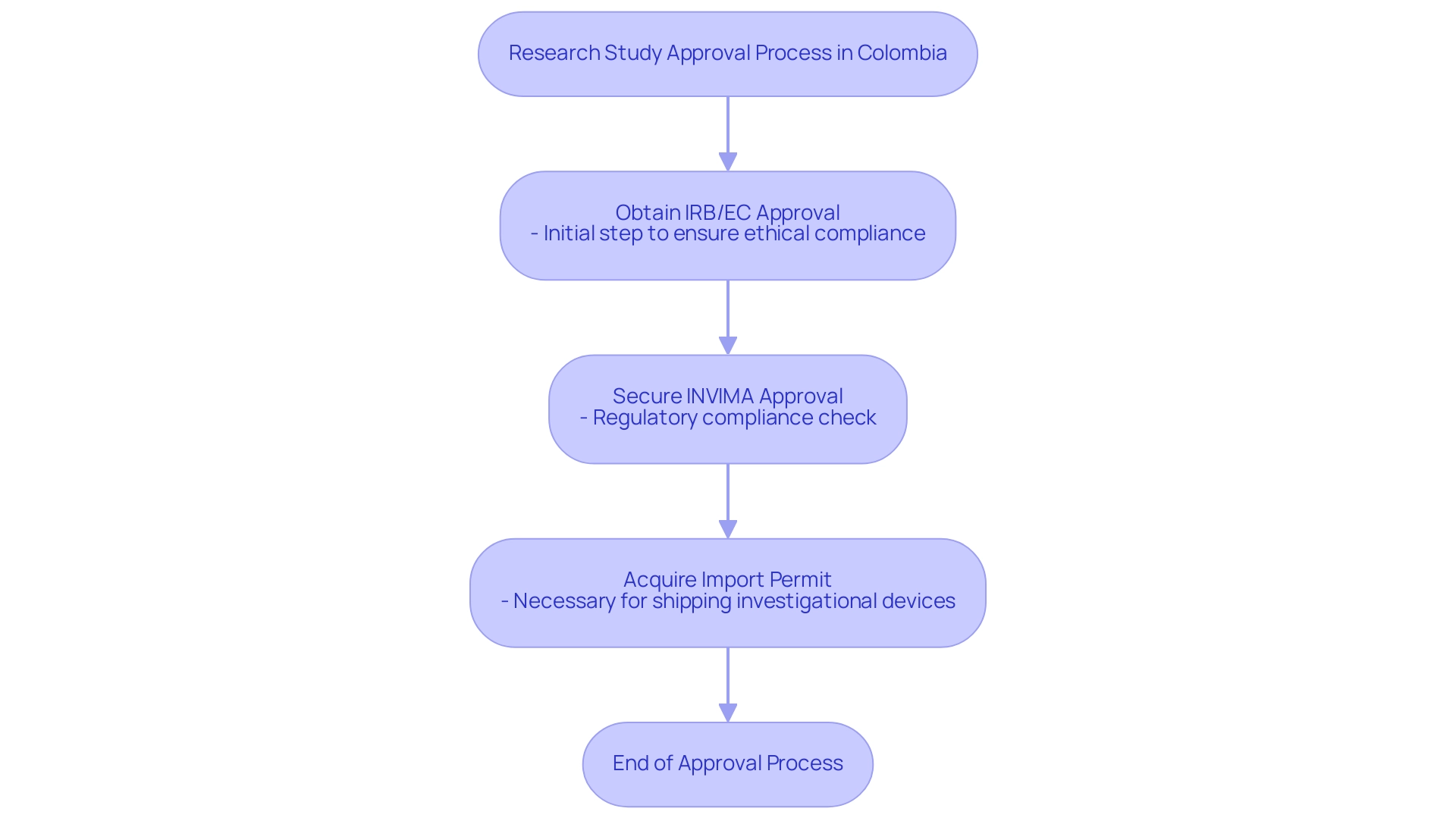
The process to obtain research study approval in Colombia involves several key steps:
- Obtaining study approval from the site's institutional review board (IRB)/ethics committee (EC)
- Securing study approval from Colombia's regulatory agency (INVIMA) and the Ministry of Health (MoH)
- Acquiring an import permit from Colombia's Ministry of Industry and Commerce (MinCIT) to ship investigational devices to the site
Adherence to these regulations is paramount; noncompliance can result in serious repercussions, including the potential invalidation of research findings. Statistics show that roughly 40% of research trials in Latin America encounter delays because of regulatory challenges, highlighting the essential role of contract research organizations in enabling prompt approvals and ethical assessments. Their deep understanding of local regulatory nuances is essential in safeguarding the integrity of clinical studies and cultivating trust among stakeholders.
Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics, exemplifies the expertise required to navigate these challenges. Her background in securing import licenses for diagnostic reagents and medical devices at INVIMA directly supports the CRO's capabilities in managing regulatory complexities. Recent adherence strategies have effectively increased registration rates, emphasizing the importance of CROs in this evolving regulatory environment.
As Urimara Argotti-Rodríguez emphasizes, there is a critical agreement among stakeholders to be accountable for all aspects of the work, ensuring that inquiries related to the accuracy and integrity of the findings are thoroughly investigated and resolved. This commitment to compliance is further highlighted by the need for Latin American regulations to adapt continually to emerging standards, necessitating frequent updates and early dialogues between health authorities and sponsors.
The examination of medical studies within the area, as outlined in the case study 'Characteristics of Medical Studies in LAC,' shows that while most are treatment-related and published mainly in English, there persists an urgent requirement for ethical adherence and the inclusion of overlooked diseases in scientific agendas. This case study analyzed 297 clinical trials, indicating that while most were randomized and blinded, only a small percentage focused on neglected diseases, emphasizing the need for a more inclusive research approach.
The Importance of Patient Recruitment and Retention
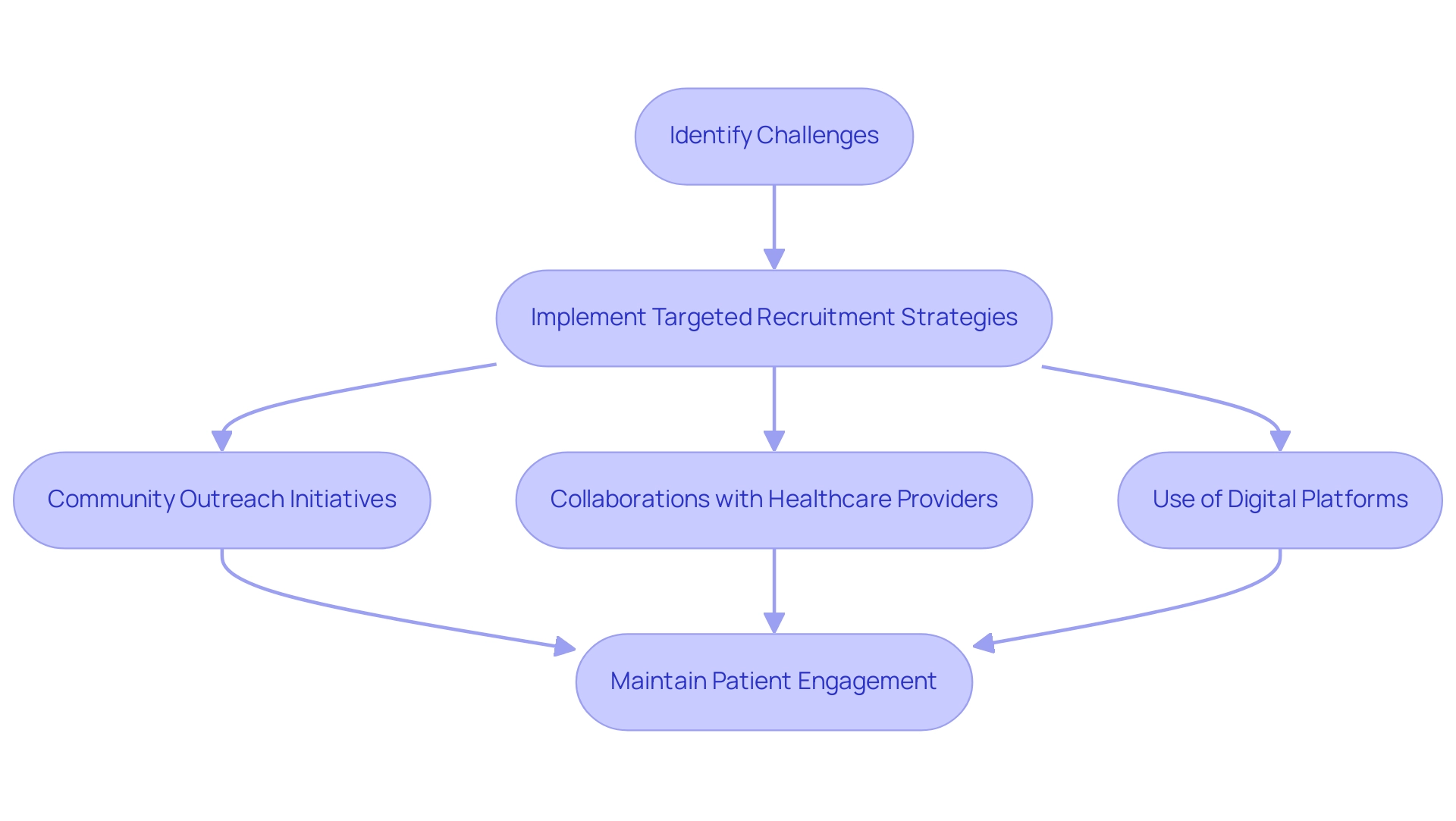
Effective patient recruitment and retention remain among the most pressing challenges for Contract Research Organizations operating in Latin America. To navigate these challenges, clinical research organizations implement targeted recruitment strategies that align with the cultural and social dynamics of local populations. According to recent statistics, patient enrollment in Latin American research studies can vary significantly, with some investigations reporting recruitment rates as low as 30% due to various barriers.
Community outreach initiatives, collaborations with local healthcare providers, and the use of digital platforms to increase awareness of ongoing research studies are common strategies employed by CROs. A notable approach involves fostering relationships that improve subject enrollment and retention, as emphasized by Natalie Gershman, CEO and medical director of Geny Research, who states, "This relationship significantly helps to increase subject enrollment and retention rates, and improves patient compliance."
Moreover, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for research studies in Latin America, backed by Colombia's Minister of Health. This initiative is anticipated to promote more research projects in the region, enhancing patient recruitment efforts. Furthermore, GlobalCare Clinical Studies has collaborated with bioaccess™ to enhance its ambulatory services in Colombia, achieving over a 50% decrease in subject recruitment time and a 95% retention rate.
Contract research organizations offer extensive clinical study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study preparation
- Reporting
These services are crucial for successful studies. Maintaining patient engagement throughout the trial is paramount for ensuring high retention rates. For instance, a case study from Brazil demonstrated that personalized follow-up communications and educational resources led to a 25% increase in participant retention compared to traditional methods.
CROs frequently employ robust support systems to keep participants informed and motivated. This dual focus on recruitment and retention not only enhances the quality and reliability of research outcomes but also tackles the unique challenges presented by varying patient demographics across the region. Present obstacles in patient retention involve language barriers and cultural misunderstandings regarding research studies, which can impede participant adherence. However, cultural acceptance of medical study participation enhances compliance, as observed in case studies from nations with efficient regulatory systems, such as South Korea and Singapore.
By prioritizing these strategies, clinical research organizations position themselves to overcome obstacles and achieve successful outcomes. Moreover, the influence of medical studies on local economies is substantial, generating employment, fostering economic development, and enhancing healthcare services, which further motivates involvement in research initiatives.
The Future of CROs in Latin America
The outlook for Contract Research Organizations in Latin America is exceptionally bright, driven by technological advancements and an increasing global focus on patient-centric research methodologies. As the demand for research studies increases, contract research organizations are poised to improve their extensive study management services, which encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study preparation (including acquiring import permits and ethics committee approvals)
- Project oversight
- Reporting
These capabilities are crucial for ensuring the successful execution of clinical studies while adhering to regulatory requirements.
Moreover, the impact of Medtech clinical studies on local economies cannot be overstated. By conducting these studies, clinical research organizations contribute to:
- Job creation
- Economic growth
- Healthcare improvement within their regions
This fosters international collaboration and knowledge transfer that ultimately benefits global health outcomes. For instance, the incorporation of artificial intelligence for data analysis and patient recruitment is expected to transform conventional processes, improving accuracy and efficiency.
In 2024, CROs are predicted to progressively adopt predictive analytics for study design and patient selection, optimizing resource allocation and enhancing overall study efficiency. The trend towards decentralized medical trials is also likely to gain momentum, driven by the need for enhanced patient engagement and accessibility. Case studies from organizations that have successfully implemented these strategies will illustrate their effectiveness across various study settings.
In 2023, Latin America represented 3.1% of the global in vivo CRO market, indicating substantial growth potential. As Anil Kumar P, Research Manager in Healthcare, states, 'I am committed to staying at the forefront of industry innovations, ensuring that my work consistently exceeds client expectations.' This commitment emphasizes the necessity for clinical trial organizations to maneuver through a changing environment efficiently.
While technology's influence on medical investigations promises to be revolutionary, industry specialists recommend a more unified method to data oversight and patient engagement. Furthermore, ongoing workforce training and the necessity for adapting to regulatory changes are critical for contract research organizations to remain proactive and adaptable. By embracing these trends and addressing potential hurdles, Latin American CROs can solidify their roles as vital contributors to the global clinical research arena.
Conclusion
The landscape of clinical research in Latin America is rapidly evolving, with Contract Research Organizations (CROs) playing a fundamental role in its advancement. By offering a wide range of specialized services, these organizations not only facilitate efficient trial management but also leverage local expertise to navigate complex regulatory environments. Their significant revenue share in the clinical segment highlights their importance as partners for pharmaceutical and medical device companies seeking to enhance research outcomes.
The contributions of CROs extend beyond operational support; they actively engage in patient recruitment and retention strategies that are culturally attuned to diverse populations. The success of initiatives such as partnerships with local healthcare providers and community outreach demonstrates the effectiveness of tailored approaches in improving enrollment rates and participant compliance. Moreover, the integration of advanced technologies is poised to further revolutionize clinical trials, making them more accessible and efficient.
As the demand for innovative medical solutions continues to rise, the role of Latin American CROs will only become more critical. Their ability to adapt to changing regulatory frameworks, while fostering economic growth and healthcare improvements within their regions, underscores their value in the global clinical research ecosystem. With a commitment to excellence and continuous improvement, these organizations are well-positioned to lead the charge in advancing medical knowledge and innovation, ultimately benefiting patients and healthcare systems alike.




