Introduction
The evolution of Electronic Data Capture (EDC) systems marks a pivotal moment in the realm of clinical trials, revolutionizing how data is collected, managed, and stored. As the industry shifts away from traditional paper-based methods, these digital platforms not only streamline data entry processes but also enhance accuracy and reduce the likelihood of human error. With real-time access to trial data, researchers are empowered to monitor progress and make informed decisions swiftly, significantly improving the efficiency of clinical studies.
Moreover, EDC systems play an essential role in ensuring compliance with regulatory standards while adapting to the increasing complexity of trial sponsorships. As the landscape of clinical research continues to evolve, understanding the functionalities, benefits, and emerging trends of EDC systems becomes crucial for stakeholders aiming to optimize their study designs and contribute to advancements in healthcare.
Understanding Electronic Data Capture Systems in Clinical Trials
Electronic data capture systems clinical trials represent a substantial improvement in the gathering, administration, and storage of information produced during clinical studies. Unlike conventional paper-based approaches, electronic data capture systems clinical trials enable a streamlined information entry process, significantly improving accuracy and reducing human mistakes. These electronic data capture systems clinical trials enable real-time access to experimental data, empowering researchers to monitor progress and make informed decisions promptly.
Furthermore, electronic data capture systems clinical trials play a crucial role in ensuring compliance with regulatory standards, including the necessary approvals from ethics committees and health ministries, thus safeguarding the integrity of clinical research. Recent statistics reveal that in 2022, non-industry sponsored studies represented 61.9% of clinical investigations in China, reflecting a broader trend towards more diverse sponsorship. As mentioned by industry expert Amy Silverstein, 'In this environment, the most successful clinical study sponsors will be the biopharma companies that evolve their clinical study delivery model to create a compelling value proposition for study participants and sites.'
Significantly, sponsors can carry out patient-burden protocol evaluations to enhance study design and boost recruitment rates, further emphasizing the advantages of electronic data capture systems in clinical trials. Our extensive service abilities, encompassing feasibility studies, site selection, compliance evaluations, setup for experiments, import permits, project management, and thorough reporting on study status, inventory, and adverse events, enhance the effectiveness of EDC processes. The case analysis of Medrio illustrates the efficiency of electronic data capture systems for clinical trials; the company offers a range of adaptable clinical research technologies that support conventional, virtual, or hybrid studies, enabling successful execution.
Furthermore, as Customer Success Manager, Gilbert concentrates on assisting customers in maximizing advantages by overcoming obstacles and reaching their objectives with EDC tools. As the clinical research environment keeps changing, a comprehensive grasp of electronic data capture systems clinical trials' functionality and importance becomes progressively crucial for all parties engaged in clinical studies, ultimately aiding in job creation, economic growth, and healthcare enhancement in local economies.
Key Features and Benefits of EDC Systems
Electronic data capture systems clinical trials are crucial in advancing clinical trial management, offering a comprehensive suite of key features aimed at improving information quality and operational efficiency. Central to this is the capability for automated validation of information, which ensures that all entered information adheres to predefined criteria, thereby supporting the Medicines and Healthcare products Regulatory Agency (MHRA) definition of integrity—emphasizing completeness, consistency, and accuracy. This functionality significantly mitigates the risk of errors, bolstering the integrity of the information gathered.
Furthermore, EDC frameworks include strong audit trails that enable clear monitoring of modifications, ensuring adherence to strict regulatory standards established by organizations like the FDA and MHRA. For instance, a case analysis on the merging of eSource and EDC platforms demonstrates how clinical research software can concurrently assist both, enabling researchers to leverage complementary functions within a study's information ecosystem. This integration empowers researchers to choose software that meets their immediate needs while accommodating future adaptations as studies evolve.
Moreover, EDC frameworks simplify information entry and management procedures via user-friendly interfaces, resulting in improved efficiency among research personnel. Immediate information access enables researchers to make informed choices quickly, speeding up testing schedules and ultimately enhancing patient results. Alongside our service capabilities, which encompass:
- feasibility studies
- site selection
- compliance reviews
- setup and approval processes
- import permits
- project management
- comprehensive reporting on serious and non-serious adverse events
these EDC platforms play a crucial role in advancing global health enhancement through international collaboration and innovation in medtech.
As Dr. John Smith, a clinical trial specialist, states, 'The characteristics of electronic data capture systems in clinical trials not only enhance information precision but also boost overall study effectiveness, making them essential in contemporary clinical research.' Collectively, the adoption of electronic data capture systems clinical trials represents a transformative approach in clinical research, significantly elevating the quality and reliability of the data generated.
Types of Electronic Data Capture Systems: Commercial vs. Open Source
Electronic data capture systems for clinical trials are mainly classified into two categories: commercial and open-source solutions. Commercial electronic data capture systems for clinical trials are proprietary software designed to provide a comprehensive suite of features, technical support, and adherence to regulatory standards. These frameworks are frequently defined by their user-friendly interfaces, extensive training resources, and dedicated customer support, making them especially attractive to organizations looking for strong, dependable electronic data capture systems for their clinical studies.
Industry leaders highlight that electronic data capture systems for clinical trials not only enhance information gathering but also support adherence to strict regulatory standards, which is essential in preserving the integrity of clinical investigations. For example, Crossix links anonymized health information on over 300 million patients to media resources, demonstrating the ability of commercial EDC platforms to handle extensive datasets efficiently.
In the realm of clinical trial management, our service offerings encompass:
- Feasibility and selection of research sites and principal investigators (PIs)
- Thorough review and feedback on study documents to adhere to country requirements
- Careful trial setup and approval processes involving ethics committees and health ministries
- Incorporation of electronic data capture systems in clinical trials for the administration of import permits for investigational devices
- Project management and monitoring services for electronic data capture systems in clinical trials
- Detailed reporting on study status, inventory, and both serious and non-serious adverse events
This holistic approach ensures that the integration of electronic data capture systems in clinical trials aligns with all regulatory needs, particularly in the context of INVIMA, the Colombia National Food and Drug Surveillance Institute, which oversees medical device regulations as a Level 4 health authority by PAHO/WHO.
Conversely, open-source EDC solutions offer a distinct advantage in terms of flexibility and customization. Organizations employing these frameworks can adjust the software to suit their specific research needs, permitting a customized approach to data management. However, this level of customization often necessitates a higher degree of technical expertise for effective implementation and ongoing maintenance.
As clinical experiments progress, especially with the rising occurrence of chronic illnesses and the need for innovative solutions from developing nations, the decision between commercial and open-source electronic data capture systems for clinical trials becomes increasingly crucial.
A pertinent case study is Novo Nordisk A/S, a Danish company recognized for its worldwide influence in the areas of obesity, diabetes, and hormone replacement therapy. The significance of operational efficiency in clinical studies across 80 nations is highlighted by their use of electronic data capture systems for clinical trials, stressing product accessibility and compliance with regulatory standards.
Ultimately, organizations must thoroughly assess the strengths and weaknesses of each type of platform, including electronic data capture systems for clinical trials, while considering factors such as budget limits, resource availability, and specific research needs. Incorporating insights from industry leaders can further guide this decision-making process. For instance, one expert observed, "The benefits of proprietary EDC solutions reside in their reliability and the assistance they offer, which can be vital for upholding compliance in clinical studies."
This informed decision-making process is vital for choosing the most appropriate electronic data capture systems for clinical trials solution that aligns with their operational objectives and enhances the effectiveness of clinical studies.
Best Practices for Implementing EDC in Clinical Trials
The effective execution of electronic data capture systems clinical trials relies on several best practices that enhance both functionality and compliance. First and foremost, comprehensive training designed for all users is essential; it fosters familiarity with the system's capabilities and ensures proficient information handling. Establishing clear information entry protocols and validation rules is equally crucial, as it safeguards integrity and compliance with regulatory standards.
Regular communication among team members can significantly mitigate challenges during the implementation phase, ensuring that any issues are addressed promptly. As highlighted in recent statistics, the implementation of regulatory compliant data management tools helps Clinical Data Management (CDM) teams meet increased demands for improved standards. Furthermore, performing pilot tests prior to full-scale implementation enables organizations to recognize possible challenges and enhance their processes, thereby simplifying the shift to electronic data capture systems clinical trials.
As one participant noted, 'communication between all collaborators is critical; the process could be streamlined rather than prolonged,' emphasizing the need for cohesive teamwork in executing these best practices. Lastly, ongoing support and maintenance are vital. As clinical research develops, so do the needs and regulations associated with it; consequently, a strong support framework guarantees that the EDC system stays effective and flexible throughout the study.
The importance of these practices is further reinforced by a case study titled 'Outline of Data Management in Clinical Research,' which discusses how electronic data capture systems clinical trials are essential for facilitating the collection of reliable and high-quality data, thereby accelerating the drug development process to market.
Future Trends in Electronic Data Capture for Clinical Trials
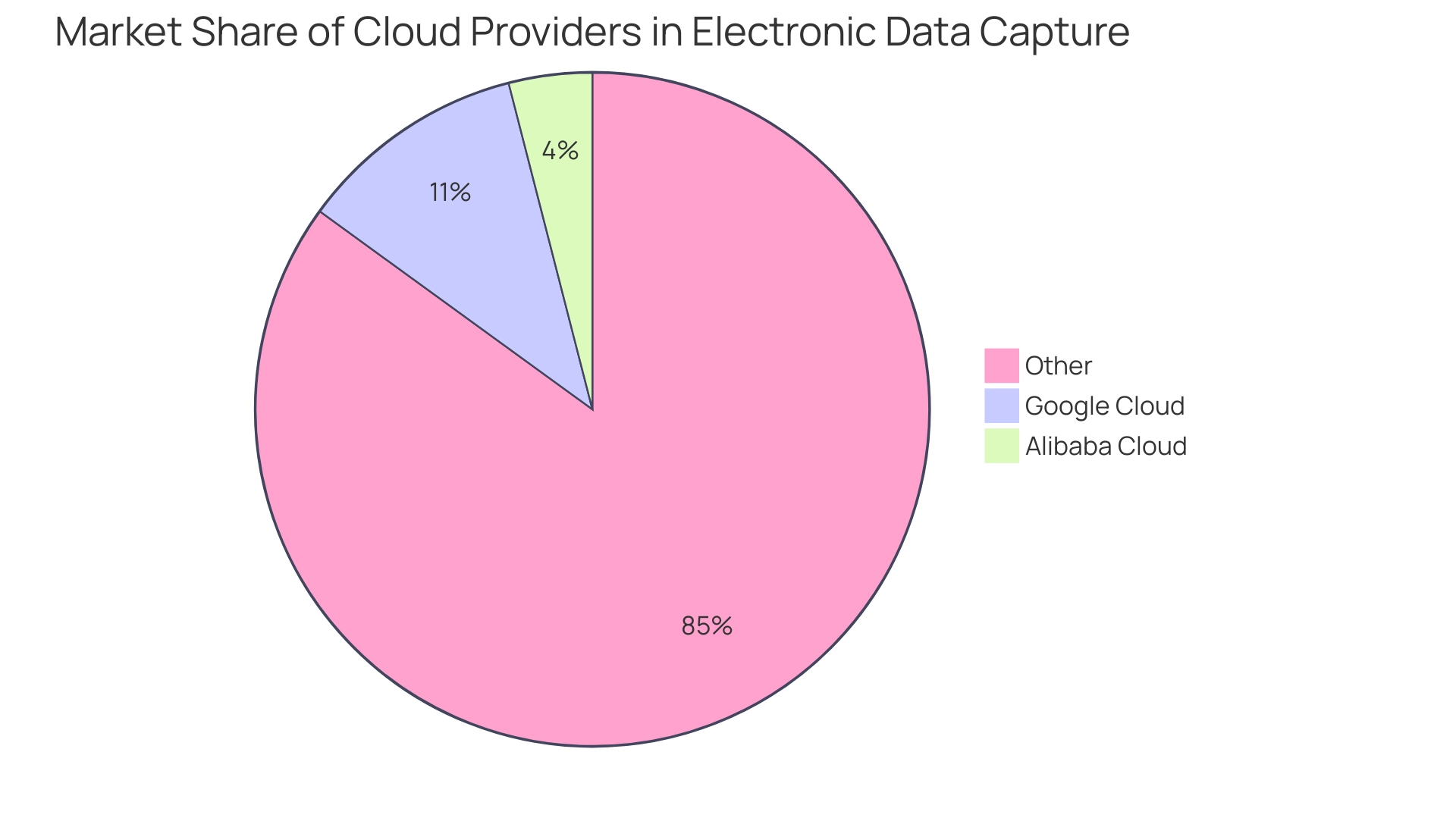
The landscape of electronic data capture systems clinical trials is undergoing significant transformation, driven by technological advancements and evolving regulatory frameworks. A notable trend is the heightened adoption of electronic data capture systems clinical trials, which provide enhanced accessibility and foster collaboration among research teams. With Google Cloud capturing an 11% market share and showcasing a remarkable 63.7% growth in its Infrastructure as a Service (IaaS) offering, the demand for such solutions is evident.
In contrast, Alibaba Cloud holds a 4% market share, indicating a competitive landscape in the cloud-based EDC market. Additionally, as PwC's 2024 Cloud and AI Business Survey highlights,
What’s often missing is a recognition that AI is becoming essential and integral to business sustainability.
This sentiment highlights the expected incorporation of artificial intelligence and machine learning within electronic data capture systems clinical trials, which is anticipated to enhance information analysis and advance predictive modeling, ultimately resulting in more effective trial designs.
Moreover, the growth of patient-focused approaches is transforming EDC development, with platforms increasingly created to support remote information gathering and improve patient involvement. This shift also raises considerations regarding sustainability, as global energy consumption by cloud data centers represents 1% of the world’s total energy use. As these trends continue to influence the future of clinical research, organizations must stay adaptable and informed to fully harness the capabilities of electronic data capture systems clinical trials.
Conclusion
The evolution of Electronic Data Capture (EDC) systems has undeniably transformed the landscape of clinical trials, offering a myriad of advantages that enhance data integrity, streamline processes, and foster compliance with regulatory standards. By moving away from traditional paper-based methods, stakeholders can now leverage real-time data access, automated validation, and robust audit trails, which collectively improve the accuracy and efficiency of clinical research. The distinction between commercial and open-source EDC systems further highlights the necessity for organizations to evaluate their specific needs and resources when selecting the appropriate solution.
Implementing EDC systems effectively hinges on best practices that ensure seamless integration and user proficiency. Comprehensive training, clear data protocols, and ongoing support are essential components that help mitigate challenges during the transition process. Additionally, the future trends within the EDC domain—such as the rise of cloud-based solutions and the integration of artificial intelligence—promise to further enhance the capabilities of these systems. As the industry continues to evolve, remaining informed and adaptable will be crucial for maximizing the potential of EDC systems in clinical trials.
Ultimately, the adoption of EDC systems is not merely a technological shift but a strategic imperative for advancing clinical research. By embracing these innovations, organizations can significantly improve trial outcomes, contribute to healthcare advancements, and better serve the needs of participants and stakeholders alike.
Frequently Asked Questions
What are electronic data capture (EDC) systems in clinical trials?
Electronic data capture systems in clinical trials are advanced tools that improve the gathering, administration, and storage of information produced during clinical studies, offering a streamlined process compared to traditional paper-based methods.
How do EDC systems enhance data accuracy in clinical trials?
EDC systems significantly improve accuracy by reducing human errors through automated information entry processes and validation checks, ensuring that all entered data adheres to predefined criteria.
What benefits do EDC systems provide for researchers during clinical trials?
EDC systems allow for real-time access to experimental data, enabling researchers to monitor progress and make informed decisions promptly, which can speed up testing schedules and enhance patient outcomes.
How do EDC systems ensure compliance with regulatory standards?
EDC systems help maintain compliance by facilitating necessary approvals from ethics committees and health ministries, and they include robust audit trails to monitor modifications, ensuring adherence to standards set by organizations like the FDA and MHRA.
What role do EDC systems play in the recruitment process for clinical trials?
EDC systems support patient-burden protocol evaluations, which help enhance study design and recruitment rates, making it easier for sponsors to attract participants.
What services are typically included with EDC systems?
Services associated with EDC systems include feasibility studies, site selection, compliance evaluations, setup for experiments, import permits, project management, and comprehensive reporting on study status and adverse events.
How do EDC systems contribute to the overall effectiveness of clinical trials?
By improving information quality and operational efficiency, EDC systems enhance the precision of data collected, ultimately boosting overall study effectiveness, as noted by clinical trial specialists.
What is the significance of the 2022 statistic regarding non-industry sponsored studies in China?
The statistic indicating that non-industry sponsored studies represented 61.9% of clinical investigations in China highlights a trend towards more diverse sponsorship in clinical trials, reflecting changes in the research landscape.
How can EDC systems adapt to the evolving needs of clinical studies?
EDC frameworks allow researchers to choose software that meets their immediate needs while accommodating future adaptations, supporting both eSource and EDC functions within a study's information ecosystem.
What is the broader impact of adopting EDC systems in clinical research?
The adoption of EDC systems represents a transformative approach in clinical research, enhancing the quality and reliability of data generated, which contributes to job creation, economic growth, and healthcare improvement in local economies.

