Overview
The article addresses the question of how to choose the right Clinical Research Organization (CRO) by highlighting essential factors such as expertise, regulatory compliance, and budget considerations. It supports this by outlining the importance of selecting a CRO with a proven track record in relevant therapeutic areas, as well as emphasizing the need for effective communication and project management to ensure successful study outcomes.
Introduction
In the ever-evolving realm of clinical research, Clinical Research Organizations (CROs) play an indispensable role in facilitating the development of new therapies. By providing a range of outsourced services—from site feasibility assessments to regulatory compliance—CROs not only streamline the research process but also enhance the efficiency and effectiveness of clinical trials.
As the demand for innovative medical solutions grows, understanding the intricacies of selecting the right CRO becomes paramount for pharmaceutical and biotechnology companies. This article delves into the essential functions of CROs, the critical factors to consider when choosing a partner, a comparative analysis of leading organizations in the field, and the future trends shaping this dynamic landscape.
Through a comprehensive exploration of these themes, it aims to equip stakeholders with the insights needed to navigate the complexities of clinical research successfully.
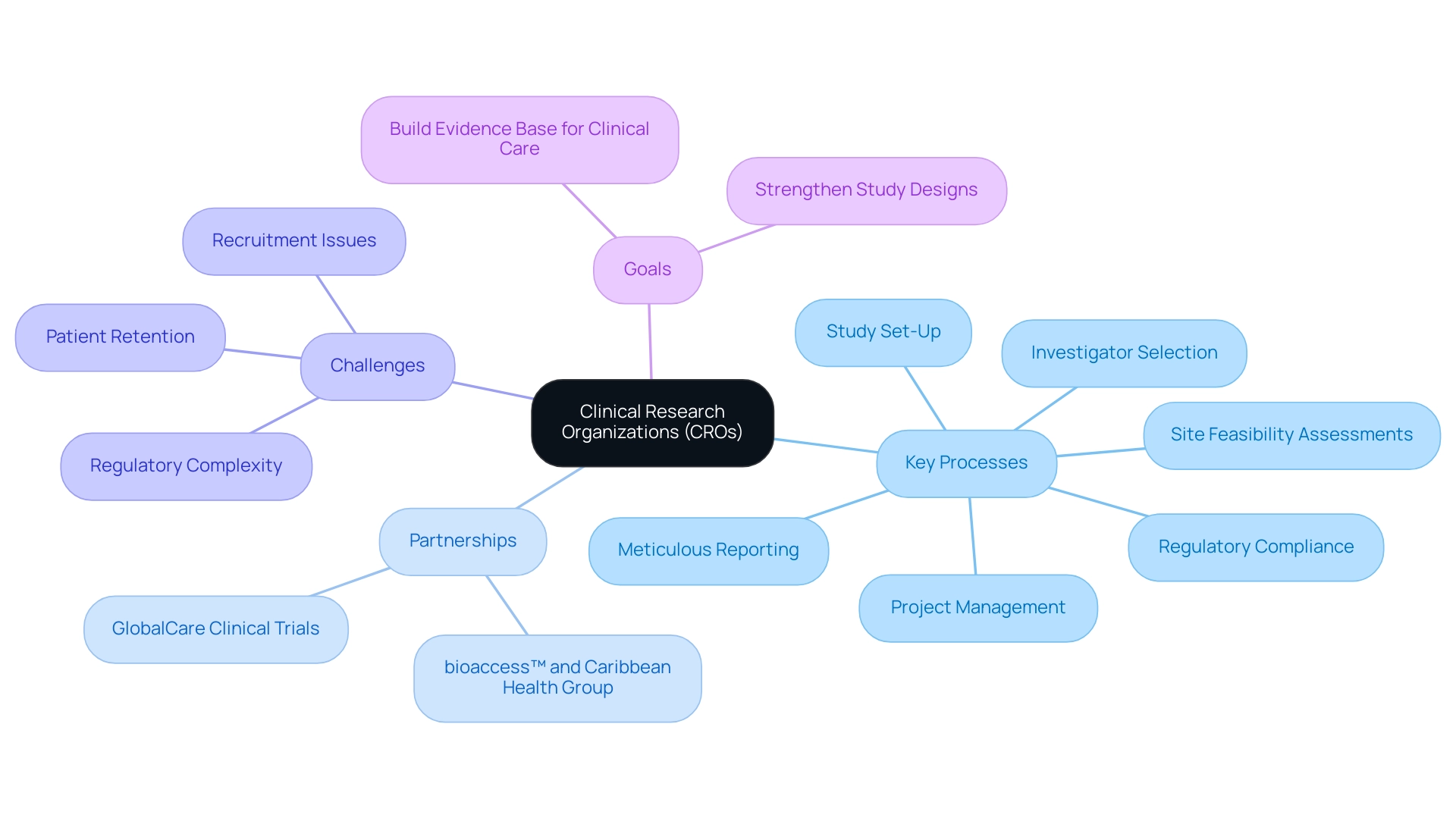
Understanding Clinical Research Organizations: An Overview
Top clinical research organizations (CROs) are crucial participants in the research landscape, offering essential outsourced services to pharmaceutical, biotechnology, and medical device firms. Their expertise encompasses comprehensive processes such as:
- Site feasibility assessments
- Investigator selection
- Study set-up
- Regulatory compliance
- Project management
- Meticulous reporting
These processes are essential for top clinical research organizations to ensure adherence to stringent ethical standards. This comprehensive approach not only streamlines the research process and reduces costs but also significantly accelerates the delivery of new therapies to market, which is essential for top clinical research organizations to address critical challenges like regulatory hurdles, competition, and recruitment issues.
Significantly, successful partnerships, like the one between bioaccess™ and Caribbean Health Group, aspire to turn Barranquilla into a leading location for clinical studies in Latin America, backed by efforts from Colombia's Minister of Health. Furthermore, partnerships like the one with GlobalCare Clinical Trials have proven effective, achieving over a 50% reduction in recruitment time and impressive 95% retention rates, highlighting the effectiveness of such collaborations. Addressing challenges such as increasing regulatory complexity and patient retention is crucial for effective management of studies.
Thus, the contributions of CROs not only advance medical knowledge but also significantly enhance patient outcomes.
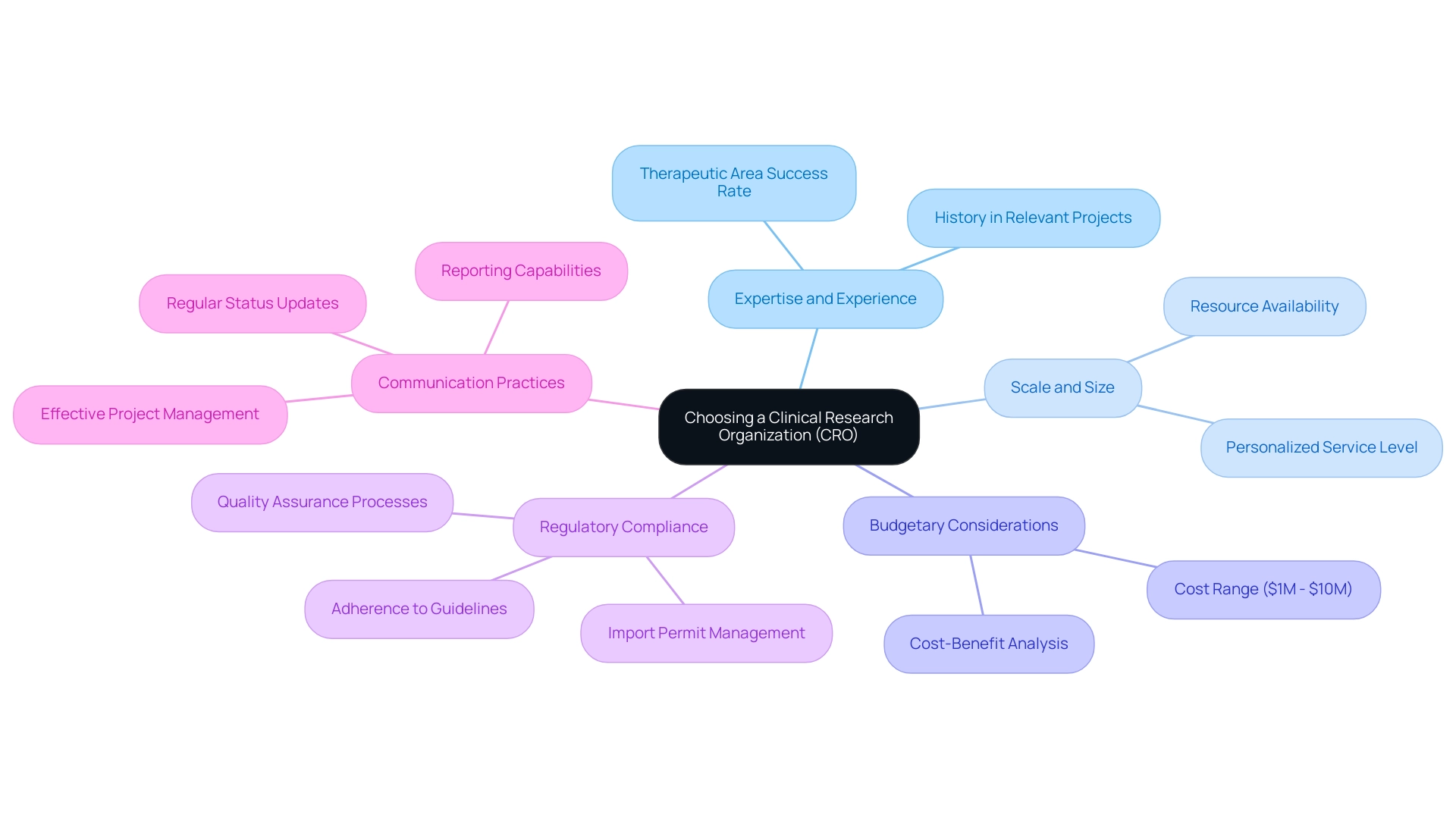
Key Factors to Consider When Choosing a CRO
Choosing the right top clinical research organizations (CRO) requires careful consideration of several key factors to ensure that the selected partner aligns seamlessly with the specific demands of the study. Foremost among these is the expertise and experience of top clinical research organizations (CROs) in managing projects similar to the one in question; those with a demonstrated history in the relevant therapeutic area can offer essential insights and operational efficiencies. Based on industry information, top clinical research organizations with substantial experience in particular therapeutic fields indicate a 20% higher success rate in studies, highlighting the significance of choosing a knowledgeable partner.
The scale and size of top clinical research organizations also play a considerable role, as they often determine the level of personalized service and attention a client may receive. Budgetary considerations are critical as well, with costs varying widely among top clinical research organizations, necessitating a thorough evaluation of the overall value that each of these organizations provides. On average, hiring a CRO can range from $1 million to $10 million depending on the complexity and duration of the study, which highlights the necessity of a cost-benefit analysis.
Moreover, understanding the commitment of top clinical research organizations to regulatory compliance and quality assurance is imperative, as adherence to established guidelines is fundamental to the integrity of clinical research. Services such as feasibility assessments, site selection, review and feedback on documents, and compliance evaluations are essential in ensuring that the research adheres to all necessary regulations. Additionally, the import permit and nationalization of investigational devices are crucial processes that should be managed effectively.
As Maria Harutyunyan noted, 'Choosing a CRO with a strong track record in regulatory compliance can significantly reduce risks associated with execution. Ultimately, evaluating the communication practices and collaborative culture of top clinical research organizations is crucial to promote a productive partnership throughout the project's lifecycle. Effective project management methodologies, including the use of agile practices and regular status updates, along with robust reporting capabilities on progress and serious and non-serious adverse events, can greatly enhance the efficiency of the process.
For instance, a case study on a successful CRO partnership revealed that effective communication strategies led to a 15% reduction in project timelines. The cumulative effect of these elements not only affects the success of research studies but also emphasizes the significance of selecting top clinical research organizations that can skillfully manage the intricacies of the research environment.
Comparative Analysis of Top CROs
In the competitive environment of top clinical research organizations (CROs), several key participants differentiate themselves through their extensive research management services and client satisfaction. For example, our capabilities include:
- Feasibility studies
- Site selection
- Compliance reviews
- Experiment setup, encompassing:
- Ethics committee approvals
- Import permits
- Project management
- Thorough reporting on study status and adverse events
This range of services not only enables effective study execution but also promotes global health enhancement through international cooperation and innovation in medical technology.
WuXi AppTec has received acknowledgment as the 2023 Global CRDMO Company of the Year by Frost & Sullivan, highlighting its dedication to excellence in research. Likewise, TFS HealthScience, established in 1996, has become an important partner for biotech and pharmaceutical firms throughout their development journey. Notably, TFS was the first CRO to adopt Medidata Detect for data surveillance and risk monitoring, reflecting its innovative approach.
This organization also achieved a Silver Rating from EcoVadis in 2024, ranking it in the top 15% for sustainability excellence, a vital consideration for many sponsors.
Conversely, Fortrea is strategically positioning itself as a 'pure-play CRO' following its decision to sell its Endpoint Clinical and Fortrea Patient Access businesses for US$345 million. As Tom Pike, Chair and CEO at Fortrea, noted, this sale will provide the company with added financial flexibility to focus on growth. This significant financial maneuver highlights Fortrea's intent to streamline its operations and enhance its core offerings, emphasizing how the diverse capabilities of top clinical research organizations illustrate the importance of aligning their strengths with specific project requirements.
Some may excel in robust patient recruitment strategies and extensive global reach, making them ideal for large-scale studies, while others may concentrate on specialized therapeutic areas, providing tailored services for niche markets. Furthermore, organizations utilizing advanced technology systems that improve data handling and examination can offer clients immediate insights, further refining the research process. Moreover, a recent guide for sponsors in 2025 stresses the significance of choosing the appropriate Oncology CRO, underscoring the necessity for innovative solutions and patient-centered studies to progress development.
By comprehensively evaluating these factors, including the strengths of top clinical research organizations and the necessity of compliance with country requirements, clients can make informed decisions that effectively support their research objectives and foster job creation and economic growth in local economies.
Future Trends in Clinical Research Organizations
The medical research landscape in Latin America is undergoing a significant transformation, shaped by the pressing challenges and innovative solutions presented by Medtech companies. These companies face multifaceted issues, including:
- Regulatory hurdles
- Limited financial resources
- Communication barriers, such as language differences and fragmentation of resources, which impede seamless collaboration with top clinical research organizations (CROs) and local hospitals.
To bridge these gaps, comprehensive services are essential, including:
- Feasibility assessments
- Site selection
- Compliance reviews that ensure adherence to local regulations
- Experimental setup involving meticulous planning and coordination
- Import permits for investigational devices
- Project management to oversee the process
- Detailed reporting on progress and adverse events.
For example, the partnership between Greenlight Guru and bioaccess™ seeks to speed up Medtech innovations and studies in the region, emphasized by PAVmed's first-in-human research in Colombia. This partnership not only underscores the commitment to innovation but also illustrates the potential for job creation and economic growth, ultimately enhancing healthcare systems and fostering international collaboration.
Furthermore, as demonstrated by Walgreens' strategic contracts for clinical trial recruitment, the integration of digital technology and patient-centric approaches is reshaping the clinical research model, with top clinical research organizations increasingly prioritizing patient experience to improve recruitment and retention rates.
Adapting to the evolving regulatory landscape, including the FDA's guidance on psychedelics, will be critical for CROs to maintain research integrity and meet the changing needs of their clients.
Conclusion
The importance of Clinical Research Organizations (CROs) in the clinical research landscape is significant, as they provide essential services that streamline the development of new therapies. By managing site feasibility assessments, regulatory compliance, and project management, CROs help address challenges faced by pharmaceutical and biotechnology companies, ultimately enhancing patient outcomes.
Selecting the right CRO involves careful consideration of critical factors such as:
- Expertise in relevant therapeutic areas
- Organizational scale
- Regulatory compliance
A CRO with a strong track record can greatly influence trial success rates, making it essential to align their capabilities with the specific needs of the study. Additionally, being mindful of the financial implications of CRO partnerships is vital for maximizing value.
The clinical research landscape is also evolving, with trends like digital integration and a patient-centric focus reshaping how trials are conducted. Innovations in regions like Latin America demonstrate the potential for economic growth and improved healthcare systems through effective CRO collaborations.
In conclusion, navigating the complexities of clinical research requires a strategic approach to selecting a CRO. By understanding their essential functions and evaluating key selection criteria, stakeholders can make informed decisions that advance research objectives while contributing to the overall enhancement of global health.
Frequently Asked Questions
What are clinical research organizations (CROs)?
Clinical research organizations (CROs) are essential participants in the research landscape that provide outsourced services to pharmaceutical, biotechnology, and medical device firms.
What services do CROs offer?
CROs offer a range of services including site feasibility assessments, investigator selection, study set-up, regulatory compliance, project management, and meticulous reporting.
Why are the processes managed by CROs important?
These processes are important to ensure adherence to stringent ethical standards, streamline research, reduce costs, and accelerate the delivery of new therapies to the market.
What challenges do CROs help address in clinical research?
CROs help address critical challenges such as regulatory hurdles, competition, and recruitment issues.
Can you give an example of a successful partnership involving a CRO?
A successful partnership example is between bioaccess™ and Caribbean Health Group, which aims to make Barranquilla a leading location for clinical studies in Latin America.
What impact did the partnership with GlobalCare Clinical Trials have?
The partnership with GlobalCare Clinical Trials resulted in over a 50% reduction in recruitment time and achieved a 95% retention rate.
What are some challenges faced by CROs in clinical research?
Challenges include increasing regulatory complexity and patient retention, which are crucial for the effective management of studies.
How do CROs contribute to medical knowledge and patient outcomes?
CROs advance medical knowledge and significantly enhance patient outcomes through their comprehensive management of clinical studies.




