Overview
The article focuses on how to choose the right contract research organization (CRO) for clinical trials by considering factors such as expertise in therapeutic areas, operational capabilities, and communication styles. It supports this by detailing essential evaluation criteria, including assessing a CRO's track record, compliance processes, and the importance of maintaining oversight and collaboration to enhance project outcomes.
Introduction
The landscape of clinical research is rapidly evolving, with Contract Research Organizations (CROs) at the forefront of this transformation. These specialized entities provide essential services that streamline the execution of clinical trials, particularly in the realm of medical devices. Their expertise encompasses a wide range of functions, from regulatory compliance to patient recruitment, enabling sponsors to navigate the complexities of clinical research with greater efficiency.
As organizations increasingly turn to CROs for their extensive knowledge and resources, understanding how to select the right partner becomes paramount. This article delves into:
- The critical factors influencing CRO selection
- The advantages and challenges of collaboration
- Emerging trends in the industry
- Highlights of some of the leading CROs poised to shape the future of clinical trials
Through this exploration, stakeholders can better appreciate the vital role CROs play in advancing medical research and improving patient outcomes.
Understanding Contract Research Organizations: An Overview
Top contract research organizations (CROs) play a crucial role in the research field, offering a complete range of services that assist in the implementation of studies, especially for first-in-human medical devices in areas such as Colombia. These specialized firms serve as a top contract research organization, delivering critical expertise in various areas, including:
- Study design
- Protocol writing
- Investigator's brochure production
- Subject informed consent
- Feasibility studies
- Investigator/site selection
- Regulatory compliance
- Submissions to ethics committees and health authorities
- Data management
- Patient recruitment
Such expertise is essential for navigating the challenges medical device startups face, including regulatory hurdles and competition.
For example, GlobalCare Clinical Trials (GCCT) has collaborated with bioaccess™ to improve its ambulatory services in Colombia, achieving over a 50% reduction in research subject recruitment time and an impressive retention rate of over 95%. This partnership exemplifies how CROs can significantly impact study efficiency and effectiveness. Additionally, IDx Technologies has collaborated with bioaccess™ for a data-licensing initiative in Latin America, leveraging AI-driven technology to improve disease detection in ophthalmology.
Such partnerships highlight the significance of CROs in improving study implementation and adjusting to technological progress. Moreover, by outsourcing various components of the research process to a top contract research organization such as CROs, sponsors—including pharmaceutical companies, biotechnology firms, and academic institutions—can focus on their core competencies while benefiting from the extensive knowledge and resources that a top contract research organization provides. When choosing a CRO partner, it is essential to emphasize attributes like reliability, a patient-focused strategy, and cooperative involvement, which ultimately improves the quality and efficiency of studies and guarantees adherence to strict regulatory standards.
How to Choose the Right CRO for Your Clinical Trials
Choosing the appropriate top contract research organization (CRO) for research studies necessitates a multifaceted strategy, taking into account various essential elements. Foremost is the expertise of the top contract research organization in the relevant therapeutic area, as this knowledge can significantly influence the success rate of clinical studies. For example, a proven track record of successful experiments is essential, serving as a testament to the organization's capabilities and reliability.
In 2019, Lambda Therapeutic Research expanded its portfolio through the acquisition of Novum Pharmaceutical Research Services, recognized as a top contract research organization with a rich history of over 50 years in the field. This acquisition not only highlights Lambda's commitment to enhancing its service offerings but also underscores the importance of established experience in supporting the pharmaceutical industry in achieving its research goals effectively.
Moreover, it is vital to assess the CRO's operational capabilities, including their feasibility and selection of research sites, principal investigator (PI) qualifications, project management processes, and technological resources. Essential to this assessment are the processes involved in setup, such as obtaining ethics committee and health ministry approvals, which ensure compliance with regulatory requirements.
Additionally, the import permit and nationalization of investigational devices are essential components of the CRO's service offerings. As Chris Rush aptly notes,
Keep in mind, working with a third-party doesn’t necessarily mean handing over your entire operational tasks and then forgetting about it,
suggesting the importance of maintaining oversight and collaboration.
Furthermore, the CRO's communication style and cultural fit with the sponsor's organization can greatly enhance collaboration and project outcomes. Conducting thorough due diligence, including seeking testimonials from previous clients and reviewing feedback on study documents for compliance, can yield valuable insights into a CRO's reliability and overall performance. It is also important to consider the reporting processes, such as study status updates and the management of serious and non-serious adverse events, which are critical for maintaining transparency throughout the research.
Potential partners should consider options to request a demo and subscribe for updates, ensuring they are well-informed about the CRO's capabilities and services. This thorough evaluation process not only helps in choosing a top contract research organization but also encourages a more effective partnership that aligns with the sponsor's project goals.
Pros and Cons of Partnering with Leading CROs
Collaborating with a top contract research organization presents a host of advantages, prominently including access to specialized expertise, cutting-edge technologies, and an extensive network of resources. These organizations play a crucial role in handling the complexities of medical studies, greatly reducing the burden on sponsors by overseeing logistics and ensuring compliance with strict regulatory standards. bioaccess®, for example, specializes in extensive study management services, including:
- Feasibility and selection of research site and principal investigator
- Compliance reviews
- Setup
- Import permits
- Project management
- Meticulous reporting
As of January 2022, roughly 40,901 studies were registered for medical devices, emphasizing the growing need for effective collaborations with a top contract research organization to navigate this landscape.
However, potential drawbacks must be acknowledged. The high costs associated with CRO services can impose significant strain on budgets, particularly for smaller organizations that may struggle to absorb these expenses. Furthermore, challenges such as misalignment in communication and expectations can result in project delays or compromised quality.
Experts emphasize that the sector is struggling with numerous challenges, including:
- Strict regulatory standards
- Elevated labor costs
- Economic instability
- Data privacy issues
- The growing intricacy of study designs
These challenges can complicate CRO partnerships. As a result, it is crucial for sponsors to establish clear goals and encourage open lines of communication throughout the partnership.
Additionally, the industry is witnessing a significant transition from generalist to top contract research organizations, influenced by the growing intricacy of research studies. This transition enables a more customized approach, ensuring that research studies are carried out efficiently and effectively, tackling specific challenges with the necessary expertise. This shift is exemplified in the case study detailing how specialized top contract research organizations, like bioaccess®, align their skills and resources to meet the unique demands of early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies, ultimately leading to better outcomes.
By strategically partnering with specialized CROs, sponsors can enhance their research processes while reducing risks related to project execution, thus benefiting local economies through job creation and healthcare enhancement. For instance, studies have shown that effective CRO partnerships can lead to a 20% increase in local job opportunities and a significant boost in healthcare service accessibility, demonstrating the broader economic benefits of these collaborations.
Emerging Trends and Innovations in Contract Research Organizations
The top contract research organization (CRO) industry is undergoing significant transformation, driven by technological advancements and a heightened focus on patient-centric methodologies. Our service capabilities are at the forefront of this evolution, encompassing:
- Feasibility studies
- Selection of research sites and principal investigators
- Compliance reviews to ensure adherence to regulatory standards
- Study setup and start-up processes
- Import permits for investigational devices
- Project management
- Comprehensive reporting on study status, inventory, and both serious and non-serious adverse events
Significantly, artificial intelligence (AI) and machine learning are being systematically incorporated into research study processes.
These technologies enhance data analysis and optimize patient recruitment strategies, leading to better results. Furthermore, the emergence of decentralized trials, propelled by telemedicine and remote monitoring technologies, is revolutionizing study designs, making participation more accessible and convenient for patients. This shift is reflected in the rising Phase III success rates, which have increased to 66%, surpassing the previous 10-year pre-pandemic average of 56%.
The integration of these innovations not only enhances operational efficiency but also fosters greater patient engagement and improves data quality. For instance, Precision for Medicine, which employs over 3,000 professionals and specializes in oncology and rare diseases, recently expanded its capabilities through the acquisition of Baseline Controls. This strategic move aims to accelerate drug development processes, thereby increasing the speed and efficiency of bringing new treatments to market.
As the top contract research organization adjusts to these emerging trends, it is set to spearhead the advancement in trials innovation for 2024 and beyond. In this context, Graham Clark, CEO of Phastar, emphasizes the importance of this evolution by stating,
A return to normality in terms of the macro environment and funding for smaller biotechs should effectively feel like growth compared to the last couple of years.
Additionally, as the industry evolves, companies like Proclinical are actively recruiting for roles such as Senior Principal Biostatistician, highlighting the growing demand for skilled professionals to support these advancements.
Proclinical's emphasis on offering trial recruitment services and workforce solutions further highlights the dynamic nature of top contract research organizations.
Top Contract Research Organizations to Consider for Your Research Needs
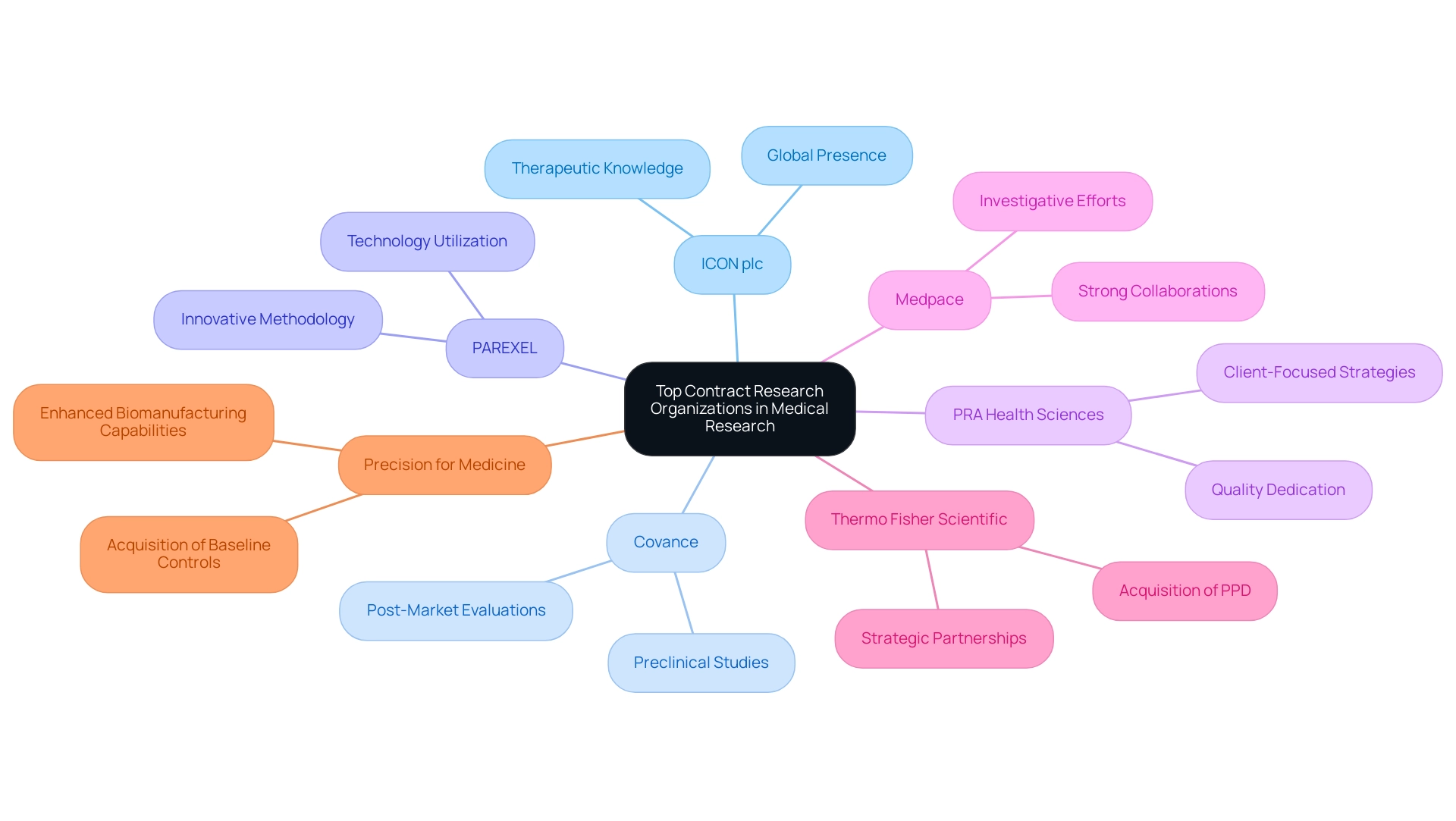
In the competitive environment of medical research, certain top contract research organizations (CROs) stand out as leaders because of their exceptional services and expertise. Among these, ICON plc distinguishes itself for its extensive global presence and profound therapeutic knowledge, making it a favored collaborator for numerous research studies. Covance offers a comprehensive suite of services, spanning from preclinical studies to post-market evaluations, ensuring a seamless continuum in drug development.
PAREXEL is especially recognized for its innovative methodology in clinical study management, utilizing technology to improve efficiency and results. Furthermore, PRA Health Sciences and Medpace have gained acknowledgment for their client-focused strategies and steadfast dedication to quality, which cultivates strong collaborations in investigative efforts. Significantly, our service capabilities encompass:
- Feasibility and selection of study sites and principal investigators (PIs)
- Thorough review and feedback on study documents to ensure compliance with country requirements
- Comprehensive trial setup and approvals through ethics committees and health ministries
Furthermore, we facilitate the import permits and nationalization of investigational devices, alongside ongoing study project management and monitoring, which includes:
- Detailed reporting on study status
- Inventory
- Both serious and non-serious adverse events
These performance metrics reflect the impactful contributions of these organizations, with accolades and success stories underscoring their roles as invaluable allies in navigating the complexities of clinical studies. As we look towards 2024, the top contract research organizations to watch include both established leaders and emerging companies, highlighting a dynamic industry landscape.
Notably, Thermo Fisher Scientific's acquisition of PPD in 2021 represents a significant shift in the CRO sector, highlighting the trend of consolidation and strategic partnerships. Michelle Keefe, CEO of Syneos Health, remarked, 'This kick-started a new era for Syneos Health as a data- and insights-driven organization, addressing market opportunities with a product development mindset.' Furthermore, Precision for Medicine's acquisition of Baseline Controls exemplifies how CROs are enhancing their capabilities to drive efficiency in drug development.
As the industry evolves, these CROs are well-positioned to meet diverse research needs, supported by robust infrastructures and a focus on advancing therapeutic development.
Conclusion
The role of Contract Research Organizations (CROs) in clinical research cannot be overstated. They provide a comprehensive range of services that are essential for the successful execution of clinical trials, particularly in the medical device sector. By understanding the critical factors influencing CRO selection, the advantages and challenges of partnerships, and the emerging trends within the industry, stakeholders can make informed decisions that significantly impact research outcomes.
The advantages of collaborating with leading CROs include:
- Access to specialized expertise
- Advanced technologies
- A vast network of resources that streamline the complexities of clinical trials
However, it is essential to recognize the potential drawbacks, such as:
- High costs
- Communication challenges
These drawbacks necessitate a clear definition of objectives and open lines of communication.
As the industry continues to evolve, with a marked shift towards specialized CROs and the integration of innovative technologies like AI and decentralized trials, the path forward becomes increasingly promising. The emerging trends highlight the need for adaptability and forward-thinking strategies in clinical research, ensuring that the focus remains on improving patient outcomes while navigating regulatory complexities.
In conclusion, selecting the right CRO is a pivotal decision that can enhance the efficiency and effectiveness of clinical trials. By prioritizing dependable partnerships and leveraging the capabilities of leading organizations, sponsors can optimize their research processes and contribute to advancements in medical innovation. As the CRO landscape continues to evolve, staying informed about industry trends and best practices will be crucial for achieving successful research outcomes.
Frequently Asked Questions
What services do top contract research organizations (CROs) provide?
Top CROs offer a complete range of services including study design, feasibility studies, regulatory compliance, data management, and patient recruitment.
Why are CROs important for medical device startups?
CROs provide critical expertise that helps startups navigate regulatory hurdles and competition, ultimately improving study implementation and efficiency.
Can you give an example of a successful collaboration involving a CRO?
GlobalCare Clinical Trials (GCCT) collaborated with bioaccess™ to enhance ambulatory services in Colombia, reducing research subject recruitment time by over 50% and achieving a retention rate of over 95%.
What should sponsors consider when choosing a CRO partner?
Sponsors should emphasize attributes such as reliability, a patient-focused strategy, and cooperative involvement to improve study quality and efficiency while ensuring regulatory compliance.
How does the expertise of a CRO influence clinical study success?
The CRO's expertise in the relevant therapeutic area and a proven track record of successful experiments can significantly affect the success rate of clinical studies.
What operational capabilities should be assessed in a CRO?
Important operational capabilities include feasibility and selection of research sites, principal investigator qualifications, project management processes, and technological resources.
What regulatory processes are essential when working with a CRO?
Essential processes include obtaining ethics committee and health ministry approvals, as well as managing import permits and the nationalization of investigational devices.
How important is communication style and cultural fit between a sponsor and a CRO?
The CRO's communication style and cultural fit with the sponsor's organization are crucial for enhancing collaboration and improving project outcomes.
What steps can potential partners take to evaluate a CRO's reliability?
Conducting thorough due diligence, seeking testimonials from previous clients, and reviewing feedback on study documents for compliance can provide valuable insights into a CRO’s reliability.
What processes should be in place for maintaining transparency during research?
Effective reporting processes, including study status updates and management of adverse events, are critical for maintaining transparency throughout the research process.

