Overview
Cost-efficient medical device trials are attainable through strategic study design, effective patient recruitment, and strict adherence to regulatory requirements. This article underscores the critical importance of implementing adaptive trial designs, leveraging technology for data management, and engaging stakeholders early in the process. Such approaches can significantly reduce costs and enhance trial success. Ultimately, these strategies pave the way for more innovative medical solutions to reach the market efficiently, demonstrating the need for collaboration and proactive engagement in clinical research.
Introduction
In the ever-evolving landscape of medical device trials, the quest for cost efficiency has become paramount. The financial stakes are rising, and the regulatory environment is growing increasingly complex. Organizations must navigate a myriad of challenges to ensure successful outcomes.
From innovative trial designs to strategic patient recruitment, optimizing resources while adhering to stringent compliance standards is essential. As the industry shifts towards embracing technology and early feasibility studies, significant cost savings and improved trial efficiency emerge.
This article delves into multifaceted strategies that can enhance the effectiveness of clinical trials, ultimately paving the way for advancements that benefit both patients and healthcare systems.
Understanding Cost Efficiency in Medical Device Trials
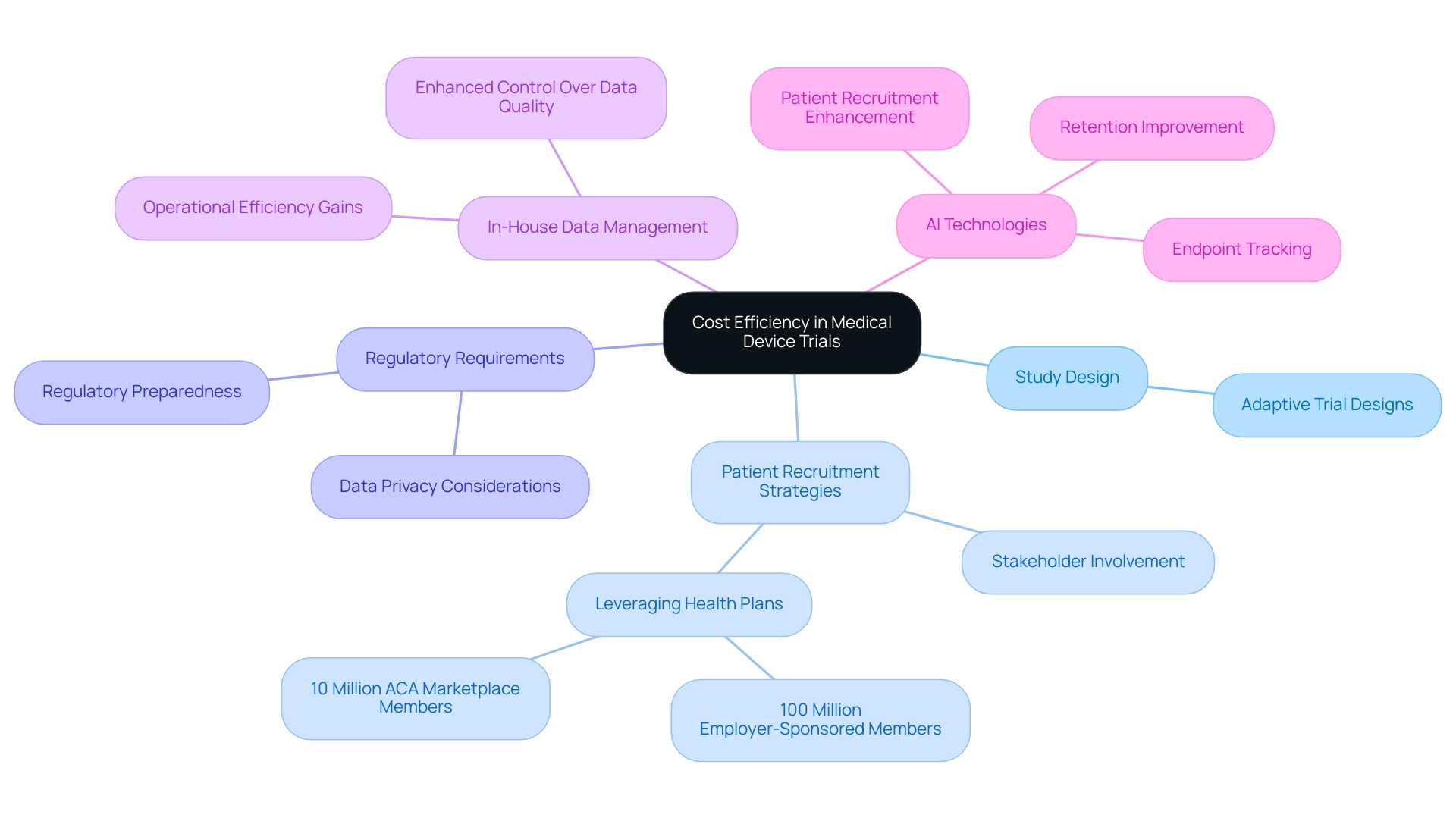
Cost-efficient medical device trials are essential for conducting research that minimizes expenses while maximizing resource utilization. Several key factors influence this efficiency, including:
- Study design
- Patient recruitment strategies
- Adherence to regulatory requirements
By strategically managing budgets and resource distribution, organizations can significantly alleviate the financial pressures associated with clinical studies.
One effective approach is the implementation of adaptive trial designs, which allow for real-time adjustments based on initial findings. This flexibility can prevent unnecessary expenditures linked to ineffective study protocols. A recent trend indicates that sponsors are increasingly insourcing management processes to enhance control over quality and operational efficiency.
This shift not only improves information management but also leads to better patient outcomes, reflecting a significant evolution in clinical information handling. As noted by a Senior Director in the Clinical Sciences and Study Management Group, "Eliminating one 20-minute task per visit across 130,000 visits avoids 43,000 hours of work. Cras can focus on what matters," highlighting the substantial efficiency gains achievable through streamlined processes.
Furthermore, early involvement with stakeholders is crucial for identifying potential cost-saving measures before the test begins. By fostering collaboration among all parties involved, organizations can streamline processes and reduce redundancies. Current statistics indicate that health plans cover approximately 100 million employer-sponsored members and 10 million Affordable Care Act (ACA) marketplace members, underscoring the vast potential for patient recruitment strategies that leverage these networks.
In 2025, the focus on cost-efficient medical device trials is more pertinent than ever, with regulatory preparedness becoming increasingly critical due to the complexities and demands of compliance, including data privacy considerations. Organizations like bioaccess®, with over 20 years of experience in Medtech, are well-positioned to navigate these challenges effectively, leveraging their expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies to ensure successful study outcomes. By adopting innovative strategies and leveraging AI technologies, bioaccess® enhances patient recruitment, retention, and endpoint tracking, making clinical research more adaptive and cost-effective.
These advancements not only contribute to successful testing outcomes but also ensure that medical devices reach the market more efficiently, ultimately benefiting patients and healthcare systems alike.
The Role of Regulatory Compliance in Cost-Effective Trials
Regulatory compliance serves as a fundamental pillar for the success of medical device studies. Adhering to the stringent guidelines established by regulatory authorities such as the FDA and EMA is essential to avoid costly setbacks that can derail progress. Studies indicate that regulatory setbacks can inflate research costs by as much as 30%, underscoring the necessity for meticulous planning and adherence to guidelines.
Organizations must prioritize comprehensive training for their teams, ensuring that every aspect of the process aligns with regulatory standards. Interacting with regulatory experts early in the process can yield invaluable insights into potential compliance challenges, enabling proactive modifications to designs. For instance, integrating feedback from regulatory agencies during the planning phase not only streamlines the approval process but also minimizes the risk of expensive amendments later on. Moreover, taking geographical factors into account is essential; areas recognized as cost-efficient for research, like Latin America, can impact both regulatory compliance expenses and overall study success.
Colombia, especially, provides competitive benefits such as regulatory efficiency, high-quality healthcare, and R&D tax incentives, making it an appealing location for first-in-human clinical studies. The financial implications of regulatory compliance are significant, and as the landscape of cost-efficient medical device trials evolves in 2025, staying abreast of the latest FDA and EMA regulations will be crucial for maintaining compliance and ensuring the successful advancement of innovative medical technologies. As noted by the Head of Clinical Information Engineering, 'Traditionally, management was outsourced to our CRO vendor partners.' Part of the initiative is to bring all our research in-house so that our internal teams can start working on it.
They can be more hands-on, and we implement research in-house and we are able to take control of our data, and we deliver for our patients with high quality. Additionally, stakeholders in research are encouraged to prepare for the anticipated final rule on single IRB review this year, which will require adjustments to standard operating procedures and resource allocation for some institutions. This proactive strategy is demonstrated by bioaccess's comprehensive clinical study management services, which encompass feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. Furthermore, bioaccess is equipped to manage the import permit and nationalization of investigational devices, ensuring a smooth setup process.
The Flow-FX study, for instance, aims to evaluate the effectiveness of the Flow-Screw device for intraosseous antibiotic delivery, showcasing bioaccess's pivotal role in advancing medical technology in Colombia.
Leveraging Early Feasibility and Pilot Studies for Cost Savings
Initial feasibility assessments (EFS) and pilot projects are pivotal components of the research landscape, particularly within the medical device industry. These investigations provide researchers the opportunity to evaluate hypotheses and gather preliminary data on device efficacy and safety before advancing to larger, more resource-intensive experiments. By pinpointing potential challenges early on, organizations can make informed decisions that significantly reduce both time and financial investments.
For instance, the average cost of a complete clinical study in the U.S. ranges from $30 to $50 million, with an estimated expense of approximately $36,500 per participant across all phases. This underscores the importance of preliminary research, which can uncover unexpected issues that might otherwise lead to costly setbacks in critical experiments. By refining experimental designs based on insights gained from preliminary tests, organizations can enhance the effectiveness of subsequent assessments, ultimately resulting in more cost-efficient medical device trials.
Moreover, leveraging advanced technologies such as artificial intelligence (AI) and machine learning (ML) algorithms can further augment these preliminary analyses. These tools can refine experimental designs and improve decision-making processes, ensuring that pilot tests yield practical insights that contribute to the overall success of medical evaluations.
As Konstantin Kalinin, Head of Content, observes, "Compliance with standards like FDA or CE involves rigorous testing and documentation, which can be both time and resource-intensive." This highlights the regulatory challenges faced in research, further emphasizing the critical role of preliminary investigations in navigating these complexities. Efficiently managing regulatory requirements can save both time and money, helping to avert costly delays in the medical device development process.
The benefits of early assessments extend beyond mere cost reductions; they also significantly impact the overall effectiveness of cost-efficient medical device trials. For example, ReGelTec, Inc. successfully enrolled eleven patients in their Early Feasibility Investigation for HYDRAFIL™ in Colombia, illustrating how such assessments can effectively gauge device performance in real-world contexts. By offering a clearer understanding of device performance and potential regulatory obstacles, EFS can streamline the development process, facilitating quicker navigation through regulatory requirements.
This proactive approach not only conserves time but also mitigates the risk of incurring additional costs associated with delays.
In summary, the strategic implementation of early viability and pilot trials is essential for medical device developers aiming to conduct cost-efficient medical device trials to enhance their research efforts. By effectively leveraging these studies, organizations like bioaccess®, with over 20 years of experience in Medtech, can pave the way for more successful evaluations and cost-efficient medical device trials, ultimately addressing the significant financial investments required for conducting studies in the U.S. Additionally, bioaccess® provides a comprehensive suite of services, including First-In-Human Studies (FIH) and Post-Market Follow-Up Studies (PMCF), to further assist clients in navigating the complexities of evaluations.
Strategies for Overcoming Recruitment Challenges in Clinical Trials
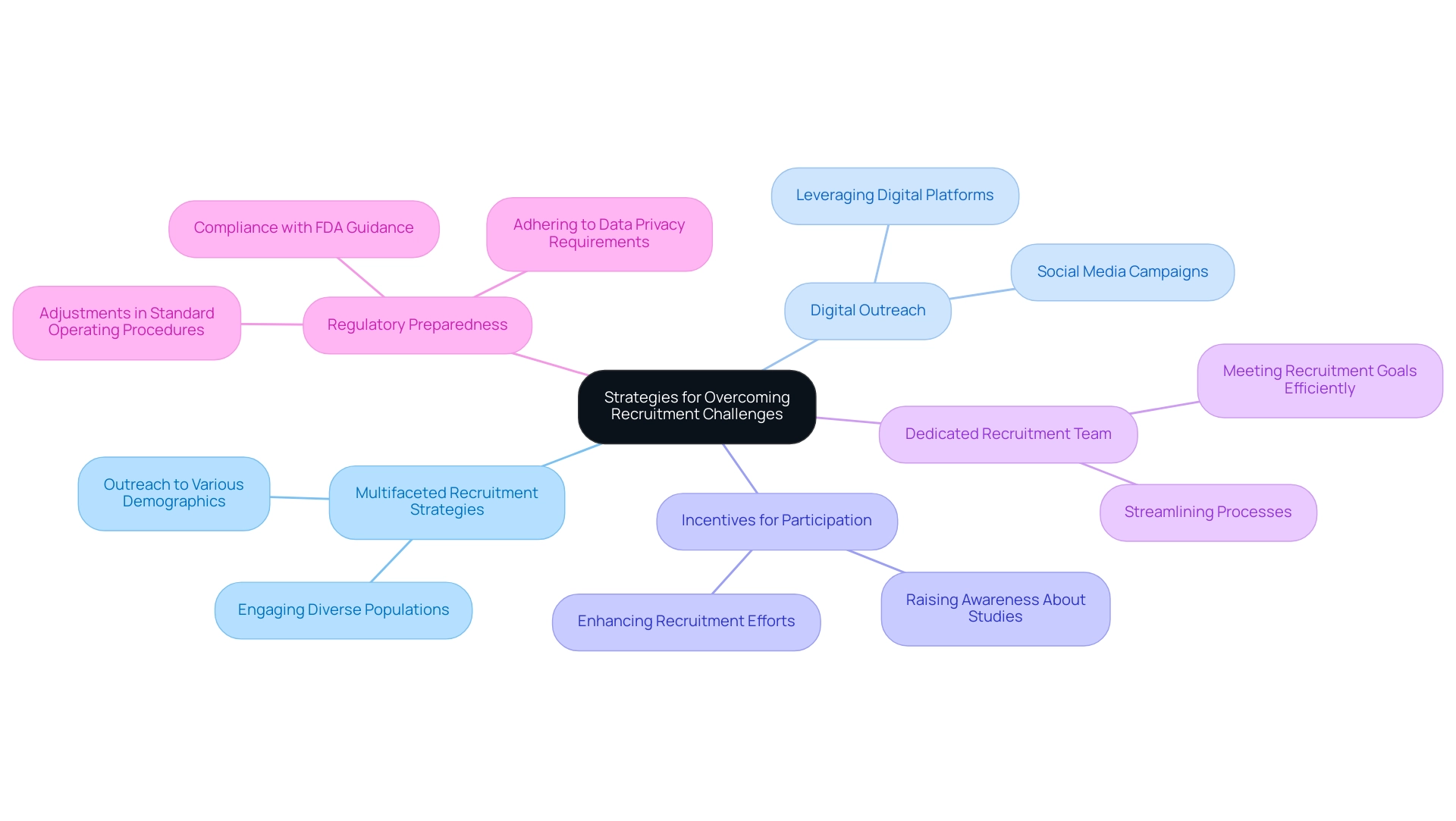
Recruitment difficulties remain a significant barrier in research studies, often resulting in delays and increased costs. As we approach 2025, the patient recruitment landscape is evolving, offering underrepresented study populations enhanced options for onboarding and visitations. This trend necessitates a reevaluation of recruitment strategies to effectively engage these diverse groups.
Organizations must adopt a multifaceted recruitment strategy that emphasizes outreach to various patient demographics. Leveraging digital platforms can substantially broaden reach, while fostering robust relationships with healthcare providers cultivates trust and encourages participant engagement.
In this context, bioaccess® offers comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. With over 20 years of experience in Medtech, their expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) positions them as a leader in effectively addressing recruitment challenges.
Incentives for participation can also be pivotal in enhancing recruitment efforts. For instance, social media campaigns have proven effective in engaging potential participants and raising awareness about ongoing studies.
Moreover, establishing a dedicated recruitment team can streamline processes and ensure that recruitment goals are met efficiently. As Bree Burks, Vice President of Site Strategy at Veeva, notes, sponsors are increasingly rethinking their site engagement strategies, underscoring the need for consistent site technology and standardization across studies. This shift is essential for overcoming recruitment difficulties and ensuring successful outcomes in cost-efficient medical device trials.
Furthermore, the collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a premier location for studies in Latin America, supported by Colombia's Minister of Health, exemplifies innovative strategies to enhance recruitment. While specific metrics from this partnership are still under evaluation, the formalization of single IRB practices will necessitate adjustments in standard operating procedures and resource allocation for certain institutions, which is crucial for improving recruitment efficiency. Additionally, regulatory preparedness is becoming increasingly vital due to complex and demanding regulations, including adherence to FDA guidance and data privacy requirements.
Addressing these elements will be essential for navigating the evolving recruitment landscape in medical studies.
Harnessing Technology to Optimize Trial Efficiency and Reduce Costs
The integration of technology in clinical trials has fundamentally transformed research methodologies, particularly in the realm of cost-efficient medical device trials. Electronic information capture (EDC) systems have emerged as a cornerstone of this evolution, significantly minimizing entry errors and enhancing the overall efficiency of data collection. In 2025, the successful implementation of EDC in medical device studies is anticipated to streamline processes, resulting in enhanced accuracy and reliability in information management.
Notably, statistics indicate that 45% of Alcon's information is entered on the same day as the visit date, exemplifying the efficiency gains afforded by EDC systems. Moreover, the adoption of remote monitoring technologies in cost-efficient medical device trials facilitates real-time data analysis, effectively reducing the necessity for on-site visits and the associated travel costs. This shift not only enhances participant engagement but also contributes to a more efficient allocation of resources. The trend towards decentralized studies (DCTs) further exemplifies this technological advancement, as these studies are recognized as cost-efficient medical device trials that utilize digital tools to connect with participants remotely, thereby reducing operational expenses and accelerating the research timeline.
In this context, bioaccess® stands out as a leader in providing accelerated medical device research services in Latin America, backed by over 20 years of experience in Medtech. Our proven track record includes managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, all with a customized approach tailored to meet the unique requirements of each study. Recent statistics suggest that organizations employing risk-based methods in trials can enhance information quality and resource efficiency, leading to cost-efficient medical device trials that ultimately reduce project timelines and expedite time to market.
A significant case analysis emphasizes the resurgence of Medical Device Regulation (MDR) solutions, which are being reassessed to better incorporate design, information gathering, analysis, and submission processes. This focus on improving metadata management and establishing a unified repository for data collection is essential for enhancing the efficiency of medical studies.
As Max Baumann, Head of Execution, stated, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." As we progress deeper into 2025, the influence of technology on research efficiency will continue to increase, with advancements in assessing outcomes anticipated to aid in the creation of medical guidelines. By embracing these innovations, organizations can not only optimize trial efficiency but also achieve significant cost savings, paving the way for cost-efficient medical device trials.
The Importance of Post-Market Clinical Follow-Up in Cost Management
Post-market clinical follow-up (PMCF) evaluations are critical for the continuous assessment of the safety and effectiveness of medical devices after their launch. These investigations yield vital information that not only directs future research and development but also promotes cost-efficient medical device trials, resulting in significant cost savings. By proactively identifying potential issues, organizations can implement corrective measures before they escalate into more serious problems that require costly interventions.
Moreover, effective PMCF strategies bolster the credibility of medical devices in the marketplace, potentially leading to increased sales and reduced marketing expenditures.
For instance, compliance with the European Union's Medical Device Regulation (MDR) mandates that manufacturers establish a post-market surveillance system tailored to the risks associated with their devices. This requirement underscores the importance of PMCF evaluations in maintaining CE marking and ensuring that devices meet safety and performance standards. Bioaccess® possesses the expertise and customized approach necessary to navigate these complexities, ensuring compliance with MDR requirements for post-market monitoring.
In addition to PMCF studies, bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pivotal Studies, leveraging over 20 years of experience in Medtech to deliver comprehensive clinical trial services.
Looking ahead to 2025, successful PMCF strategies will increasingly focus on real-world information gathering and analysis, as outlined in the MDCG 2020-7 guidance document. This approach not only aligns with regulatory expectations but also supports long-term safety monitoring, which is essential for sustaining market confidence in medical devices. For example, 45% of Alcon's data is entered on the same day as the visit date, illustrating the efficiency of real-time data management in PMCF studies.
Furthermore, manufacturers are obligated to report to the FDA when they become aware that any of their devices may have caused or contributed to a death or severe injury. This regulatory obligation highlights the critical nature of monitoring device safety and effectiveness. Organizations must prioritize the development of robust PMCF strategies, like those offered by bioaccess®, to ensure ongoing compliance and optimize resource allocation. Ultimately, this fosters cost-efficient medical device trials and cultivates a safer, more effective medical technology landscape in Latin America.
Implementing Proven Strategies for Successful and Cost-Effective Trials
Achieving success in cost-efficient medical device trials necessitates a multifaceted approach grounded in proven strategies. Organizations must understand and integrate key principles of cost efficiency into every phase of the trial process. This includes ensuring strict regulatory compliance, which is essential for maintaining the integrity of the research and facilitating smoother approvals.
Leveraging early feasibility studies (EFS) is crucial, as they enable the identification of potential challenges early in the development process, ultimately saving time and resources. bioaccess® specializes in managing:
- Early Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Providing the expertise required to navigate these complex evaluations effectively. Additionally, proactively addressing recruitment challenges can significantly enhance the efficiency of the study.
By employing innovative recruitment strategies and utilizing patient feedback, organizations can streamline participant enrollment, which is vital for the timely completion of the research. In fact, in 2025, we will observe an increased use of study protocols directly informed by feedback from patients and advocates, underscoring the importance of incorporating patient insights into research designs.
The incorporation of technology plays a pivotal role in contemporary clinical studies. By insourcing information management and utilizing advanced analytics, sponsors can gain greater control and transparency over their information, leading to improved trial quality and outcomes. This trend is increasingly evident as organizations recognize the advantages of managing information in-house, aligning with the latest ICH guidelines.
A case study titled "ReGelTec Successfully Treats First Eleven Chronic Low Back Pain Patients with HYDRAFIL™ in Colombia" illustrates this shift, showcasing how bioaccess® facilitated the successful treatment of patients through an EFS. This emphasizes the importance of effective data management and oversight.
Continuous evaluation of experimental processes is another essential strategy. By regularly assessing performance metrics and outcomes, organizations can pinpoint inefficiencies and implement necessary adjustments. This iterative method not only improves efficiency but also aids in the overall progression of medical technology.
As noted by Peng Lu, chief medical officer of Dutch biotech Pharvaris, "Homogenizing the use of specific outcomes and outcome measures for clinical studies will support clinical guidelines development and future indirect comparisons among interventions."
Ultimately, the successful integration of these strategies will lead to more effective trials and result in cost-efficient medical device trials, fostering innovation within the medical device sector and paving the way for groundbreaking advancements that can significantly improve patient care.
Conclusion
In the dynamic realm of medical device trials, achieving cost efficiency transcends being merely an operational goal; it stands as a vital necessity for success. This article has examined the multifaceted strategies essential for enhancing clinical trial effectiveness, from the implementation of adaptive trial designs and early feasibility studies to the leveraging of technology and the improvement of regulatory compliance. Each of these components plays a critical role in streamlining processes, reducing unnecessary expenses, and ultimately accelerating the time-to-market for innovative medical devices.
The significance of strategic patient recruitment cannot be overstated. By embracing diverse recruitment approaches and fostering collaboration with healthcare providers, organizations can engage underrepresented populations more effectively, ensuring that trials are not only successful but also reflective of the broader patient community. Furthermore, the integration of advanced technologies—such as electronic data capture and remote monitoring—has revolutionized the methods of data collection and analysis, leading to substantial improvements in trial efficiency.
As the medical device industry continues to evolve, organizations must remain proactive in adapting to regulatory changes and embracing innovative methodologies. By prioritizing early feasibility studies and post-market clinical follow-up, companies can identify potential challenges before they escalate, ensuring ongoing compliance and enhancing the credibility of their products.
Ultimately, the successful implementation of these strategies will not only foster cost-effective trials but also contribute to groundbreaking advancements in patient care and healthcare systems. The path forward is unequivocal: by optimizing resources and embracing change, the medical device industry can navigate the complexities of clinical trials with confidence, paving the way for a healthier future for all.
Frequently Asked Questions
Why are cost-efficient medical device trials important?
Cost-efficient medical device trials are essential for conducting research that minimizes expenses while maximizing resource utilization, which helps alleviate financial pressures associated with clinical studies.
What factors influence the efficiency of medical device trials?
Key factors influencing efficiency include study design, patient recruitment strategies, and adherence to regulatory requirements.
What is an adaptive trial design?
An adaptive trial design allows for real-time adjustments based on initial findings, preventing unnecessary expenditures linked to ineffective study protocols.
How are organizations improving control over clinical study management?
Organizations are increasingly insourcing management processes to enhance control over quality and operational efficiency, leading to better information management and patient outcomes.
What role does stakeholder involvement play in medical device trials?
Early involvement with stakeholders is crucial for identifying potential cost-saving measures and fostering collaboration to streamline processes and reduce redundancies.
How significant is regulatory compliance in medical device studies?
Regulatory compliance is fundamental for the success of medical device studies, as adherence to guidelines established by authorities like the FDA and EMA is essential to avoid costly setbacks.
What impact can regulatory setbacks have on research costs?
Regulatory setbacks can inflate research costs by as much as 30%, highlighting the necessity for meticulous planning and adherence to guidelines.
Why is it important to interact with regulatory experts early in the process?
Engaging with regulatory experts early can provide invaluable insights into potential compliance challenges, allowing for proactive modifications to study designs.
What advantages does Colombia offer for clinical studies?
Colombia offers regulatory efficiency, high-quality healthcare, and R&D tax incentives, making it an appealing location for first-in-human clinical studies.
What is the significance of the anticipated final rule on single IRB review?
The anticipated final rule on single IRB review will require adjustments to standard operating procedures and resource allocation for some institutions, necessitating proactive strategies.
What services does bioaccess® provide for clinical study management?
Bioaccess® offers comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.

