Overview
Pilot study designs for medical devices serve as essential preliminary evaluations that assess the viability, safety, and functionality of medical instruments prior to larger clinical trials. These designs not only identify potential issues early in the development process but also significantly enhance the likelihood of success in subsequent trials. By integrating innovative methodologies and adhering to regulatory requirements, pilot studies lay a solid foundation for future research endeavors. In the dynamic Medtech landscape, understanding these preliminary evaluations is crucial for navigating the complexities of clinical research.
Introduction
In the intricate realm of medical device development, pilot studies serve a crucial role in shaping the future of healthcare innovations. These preliminary investigations, often termed feasibility studies, are meticulously designed to assess the viability of new devices prior to their progression into larger clinical trials.
By evaluating safety, functionality, and overall feasibility within a limited participant pool, pilot studies not only identify potential issues but also empower manufacturers to refine their products and study protocols.
As the landscape of medical technology continues to evolve—particularly with advancements in genomics and the integration of artificial intelligence—the significance of these studies becomes increasingly pronounced. They pave the way for efficient and effective clinical trials that have the potential to transform patient care.
Understanding Pilot Studies in Medical Device Development
Pilot study designs for medical devices, also known as feasibility assessments, are essential preliminary evaluations that determine the viability of medical instruments prior to larger clinical trials. These designs typically involve a restricted group of participants and are meticulously crafted to assess critical elements such as safety, functionality, and overall practicality of the equipment. By identifying potential issues early in the development process, pilot study designs empower manufacturers to enhance their devices and refine research protocols, significantly increasing the likelihood of success in crucial trials.
In 2025, the importance of pilot trials is underscored by the fact that 54% of biopharma respondents are actively seeking to simplify enrollment in patient-support programs. This statistic highlights the necessity for efficient research designs that can streamline participant recruitment and improve the overall feasibility of clinical trials. Furthermore, recent advancements in genomics and biomarkers, as discussed in the case analysis titled 'The Future of Personalized Medicine,' are anticipated to enhance treatment efficacy while reducing side effects.
Pilot trials can capitalize on these advancements by integrating innovative therapies that address challenges early in the development process.
At bioaccess®, with over 20 years of experience in Medtech, our expertise in managing initial trials is complemented by our comprehensive clinical trial management services, which encompass feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Expert opinions reinforce that pilot study designs for medical devices are crucial for understanding their viability. Karen Becker Witkin, a principal with the Weinberg Group, Inc., notes that these designs provide invaluable insights that can lead to necessary adjustments in equipment design or reveal logistical challenges in trial management.
For example, pilot study designs for medical devices have demonstrated how effective preliminary trials in medical technology can yield early feedback, guiding modifications that enhance the probability of success in subsequent stages.
As the landscape of medical equipment advancement evolves, particularly in Latin America, the integration of technologies such as generative AI is expected to boost operational efficiencies, further emphasizing the importance of preliminary research in navigating the complexities of clinical trials. The 2023 NIH Policy for Data Management and Sharing mandates the implementation of Data Management and Sharing Plans, which influences the design and execution of initial projects by necessitating structured approaches to data management.
In summary, pilot study designs for medical devices are not merely initial steps; they are vital to the successful development of medical equipment. By focusing on feasibility, this research lays the groundwork for more effective and efficient clinical trials, ultimately contributing to the advancement of innovative medical solutions.
Objectives and Methodologies of Pilot Studies
In medical equipment research, pilot study designs for medical devices serve essential aims, primarily focusing on evaluating the practicality of research designs, assessing recruitment strategies, and identifying potential safety issues related to the equipment. As we approach 2025, methodologies utilized in these analyses have evolved to encompass a blend of qualitative and quantitative approaches. Techniques such as structured interviews, surveys, and statistical assessments are employed to gather comprehensive data on participant responses and equipment performance.
For instance, a recent trial involving an innovative cardiac instrument monitored a select group of patients to assess both the instrument's functionality and user feedback. This approach not only provided insights into the device's operational efficacy but also highlighted user experience, which is crucial for subsequent refinements. The results of such preliminary research can considerably affect the direction of the main investigation, determining whether to halt, modify, observe closely, or proceed based on the collected data.
Furthermore, the determination of sample sizes in preliminary research has become increasingly sophisticated. Establishing sample size for preliminary assessments can be complex, particularly for proof-of-concept evaluations, and should be grounded in feasibility objectives. Utilizing a confidence interval approach allows researchers to justify sample sizes effectively, ensuring that feasibility objectives are met.
As noted by Lehana Thabane, "The authors would like to correct the number of sample size in the fourth paragraph under the heading Sample Size for Pilot Studies from '75 patients' to '289 patients'." By aligning the preliminary research's objectives with pilot study designs for medical devices, researchers can enhance the trial process, ultimately leading to more successful outcomes in the larger medical investigations that follow.
At bioaccess®, with over 20 years of experience in Medtech, our proficiency in managing preliminary investigations, along with our extensive trial management services—including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project oversight, and reporting—ensures that your medical apparatus trials are conducted efficiently and effectively in Latin America.
Navigating Regulatory Requirements for Pilot Studies
Navigating the regulatory environment for preliminary trials is essential for successful medical research in the service sector. Regulatory entities, particularly the FDA, provide comprehensive guidelines that govern the execution of these investigations, ensuring adherence to safety and ethical standards. A pivotal component of these guidelines is the requirement for informed consent from participants, which protects their rights and well-being throughout the research process.
Moreover, pilot study designs for medical devices must be meticulously crafted to meet the regulatory requirements relevant to larger clinical trials. This entails establishing specific endpoints and implementing robust safety monitoring protocols. For instance, the FDA has indicated a preference for a single effective date for regulations, contending that this approach reduces confusion and enhances compliance among researchers.
As we look ahead to 2025, the regulatory landscape for pilot projects continues to evolve, with an emphasis on improving data interoperability and consistency. The International Medical Device Regulators Forum (IMDRF) strives to promote regulatory convergence among international medical device regulators, addressing emerging challenges while enhancing public health and safety. A notable case analysis illustrates the obstacles encountered in China, where over 300 commercial hospital information systems with diverse technical structures impede effective data exchange.
This scenario underscores the necessity for standardized medical terminology and coding practices, which are vital for the successful sharing of health data and the improvement of patient care.
Furthermore, expert insights highlight the importance of semantic interoperability in laboratory data. As Michael Waters, PhD, articulates, "Simply, describing the same test the same way. By enhancing the semantic interoperability of laboratory data within and among institutions, diagnostic information can be utilized to better aid healthcare decisions." This emphasizes the need for consistent diagnostic information across institutions to enhance patient outcomes.
Additionally, the Quality Management System Regulation (QMSR) is anticipated to yield approximately $528 million in cost savings at a 3 percent discount rate, underscoring the financial implications of regulatory compliance.
As the regulatory landscape evolves, comprehending these guidelines and their implications will empower research teams to navigate the complexities of pilot study designs for medical devices effectively, ensuring that their results are both credible and actionable. With Bioaccess's expertise, backed by over 20 years of experience in Medtech, in managing experimental trials and other research endeavors in Latin America—including Early-Feasibility, First-In-Human, and Post-Market Follow-Up Trials—research teams can leverage specialized knowledge and a tailored approach to successfully navigate these challenges.
Challenges in Conducting Pilot Studies for Medical Devices
Carrying out preliminary research for medical devices in 2025 presents a multitude of challenges that significantly influence the success of medical research. A primary concern is participant recruitment; identifying eligible candidates who meet specific research criteria can be both time-consuming and complex. Recent statistics underscore the critical nature of participant retention, as logistical and personal issues frequently lead to dropouts.
Indeed, the challenges surrounding participant retention are a substantial barrier, as evidenced by the statistic that 21% of platforms focus primarily on oncology, reflecting broader trends in recruitment difficulties across various medical fields. Innovative approaches to recruitment, such as individualized care and adaptive solutions, are essential to redefine standards in this area. Nicola Armitstead, Vice President of Site Clinical Operations, underscores this point, stating, "With a focus on personalized care and adaptive solutions, we continuously redefine the standards of participant recruitment and retention in research."
Moreover, compliance with evolving regulatory requirements introduces another layer of complexity. As regulations shift, particularly within the dynamic sphere of medical technology, research may face additional hurdles if their designs lack adaptability. This situation is exacerbated by logistical challenges related to data collection and management, which can strain resources and budgets.
Insights from research professionals highlight the importance of early stakeholder engagement and the integration of technology to streamline data collection processes. For instance, recent case analyses, such as the collaboration between bioaccess™ and Caribbean Health Group, demonstrate how strategic partnerships can enhance trial services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates. bioaccess™ specializes in various research types, including:
- Early-Feasibility Research
- First-In-Human Research
- Pilot study designs for medical devices
- Pivotal Research
- Post-Market Clinical Follow-Up Research
showcasing their expertise in overseeing clinical trials for medical instruments.
Furthermore, innovative digital platforms have shown promise in enhancing recruitment and patient monitoring, although they also raise concerns regarding accessibility and ethical considerations.
Additionally, understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial, as it oversees medical product regulation and classification, ensuring compliance with national standards.
In summary, addressing these multifaceted challenges necessitates meticulous planning and proactive strategies. By harnessing technology and fostering collaboration among stakeholders, including support from Colombia's Minister of Health, researchers can navigate the intricacies of trial projects more efficiently, ultimately advancing the development of medical tools.
Best Practices for Designing Effective Pilot Studies
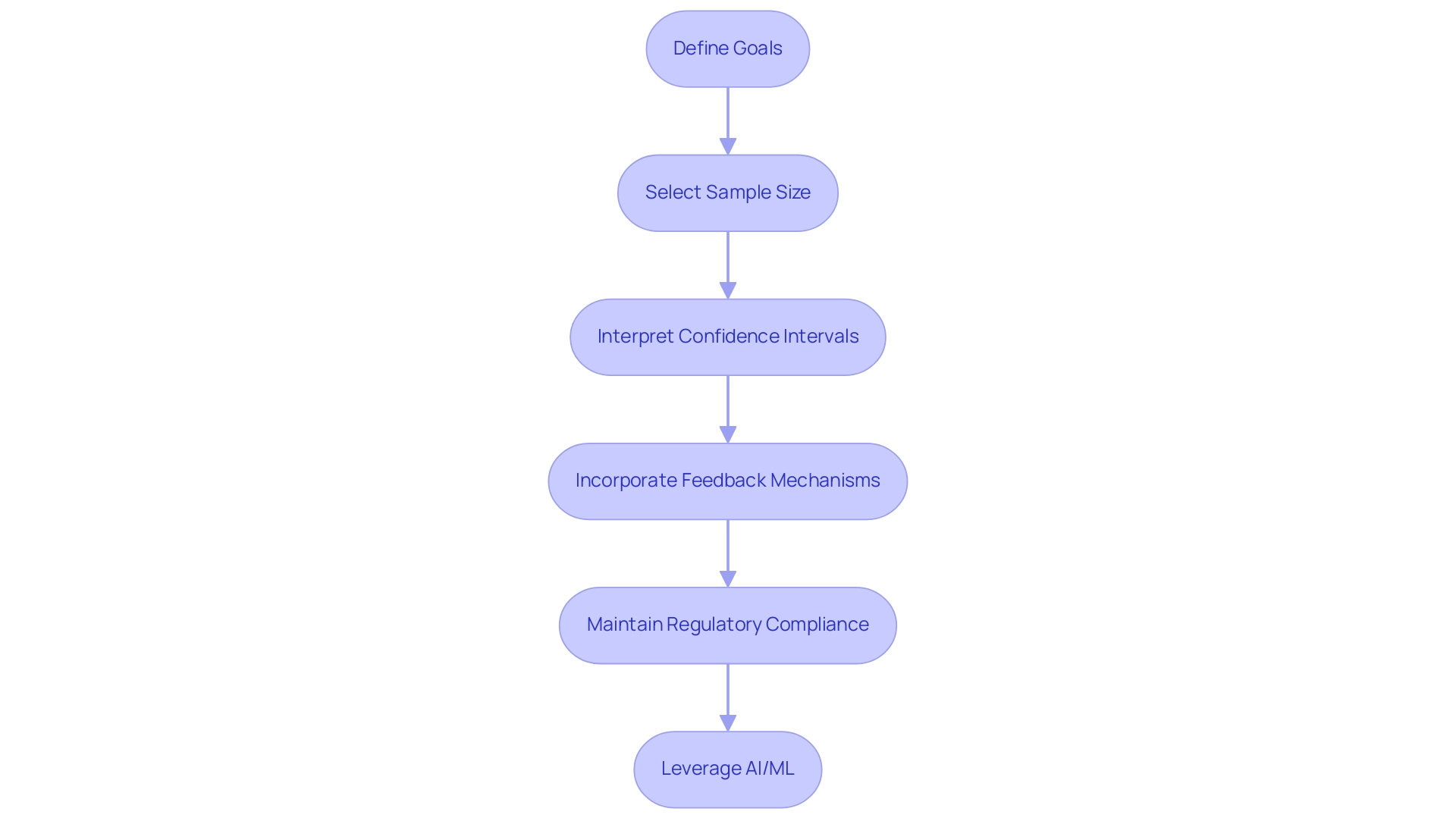
Creating efficient preliminary investigations in medical equipment research necessitates adherence to best practices related to pilot study designs for medical devices, significantly enhancing the quality and outcomes of clinical trials. First and foremost, it is essential to clearly define the goals of the trial. This involves specifying which aspects of the device or research design will be evaluated, ensuring that the evaluation aligns with broader research objectives.
Selecting an appropriate sample size is another critical factor. Research indicates that preliminary experiments with sample sizes ranging from 30 to 50 have a greater likelihood of achieving similar or enhanced retention probabilities, with a relative risk of 1.21. This statistic underscores the importance of meticulous sample size selection in pilot study designs for medical devices.
However, caution is warranted, as confidence intervals for process outcomes can be wide, particularly with smaller sample sizes. For example, a correlation coefficient with a sample size of 50 yields a confidence interval width of 0.426, illustrating the variability that can arise in pilot experiments. This variability emphasizes the need for careful interpretation of observed variance, as it can impact future power calculations.
Incorporating feedback mechanisms, such as participant interviews or surveys, is vital for gathering insights into user experience and performance. This qualitative data can inform adjustments and improvements before larger trials commence. Moreover, maintaining open communication with regulatory bodies, such as INVIMA, throughout the research is crucial for ensuring compliance in pilot study designs for medical devices and facilitating smoother transitions to subsequent phases of investigation.
INVIMA, recognized as a Level 4 health authority by PAHO/WHO, plays a significant role in medical device oversight in Colombia, making it essential for clinical research teams to align their projects with its regulatory functions.
A case analysis titled 'Sample Size for Initial Research: A Quick Guide for Biostatisticians' critiques traditional heuristics like the 'rule of 30' and advocates for newer methods that align with initial research objectives. It highlights the importance of incorporating preliminary trials into comprehensive research through pilot study designs for medical devices and blinded adaptive designs, which can enhance statistical power and ensure that preliminary data is effectively utilized in final analysis. This method not only improves the thoroughness of preliminary experiments but also guarantees that the insights gained are applicable in later research stages.
Furthermore, as pointed out by Mak, enhancing the analysis of big data with AI and ML algorithms can further refine experimental designs, enabling more nuanced insights and improved decision-making.
By following these best practices and leveraging the comprehensive clinical trial management services offered by bioaccess—including expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—along with over 20 years of experience in Medtech and an accelerated approach to clinical trials, clinical research teams can enhance the effectiveness of pilot study designs for medical devices. This ultimately leads to more successful medical device development and a faster path to commercialization.
Conclusion
Pilot studies are indispensable in the journey of medical device development, serving as the foundational assessment for feasibility, safety, and functionality before larger clinical trials commence. By engaging a limited participant pool, these studies identify potential challenges early on, enabling manufacturers to make necessary adjustments that can significantly enhance the likelihood of success in subsequent phases. With evolving methodologies and the integration of advanced technologies, pilot studies are becoming increasingly sophisticated, ensuring that data collection and participant recruitment are streamlined and efficient.
The regulatory landscape surrounding pilot studies is equally crucial, as compliance with guidelines set forth by authorities like the FDA ensures that safety and ethical considerations are met. As regulations continue to evolve, understanding and adapting to these requirements is essential for clinical research teams aiming to navigate the complexities of medical device trials effectively. The collaboration between stakeholders and the use of innovative technologies can further mitigate the challenges associated with participant recruitment and retention, highlighting the need for a proactive approach in study design.
Ultimately, the significance of pilot studies cannot be overstated. They not only lay the groundwork for successful clinical trials but also contribute to the advancement of innovative medical solutions that can transform patient care. By adhering to best practices, maintaining open communication with regulatory bodies, and leveraging technological advancements, clinical research teams can enhance the effectiveness of their pilot studies, ultimately accelerating the path toward commercialization and improving health outcomes for patients worldwide.
Frequently Asked Questions
What are pilot study designs for medical devices?
Pilot study designs, also known as feasibility assessments, are preliminary evaluations that determine the viability of medical devices before larger clinical trials. They assess critical elements such as safety, functionality, and practicality.
Why are pilot studies important in medical device development?
Pilot studies help identify potential issues early in the development process, allowing manufacturers to enhance their devices and refine research protocols, which increases the likelihood of success in larger clinical trials.
What percentage of biopharma respondents are seeking to simplify enrollment in patient-support programs by 2025?
In 2025, 54% of biopharma respondents are actively seeking to simplify enrollment in patient-support programs.
How do recent advancements in genomics and biomarkers impact pilot trials?
Advancements in genomics and biomarkers are expected to enhance treatment efficacy and reduce side effects, allowing pilot trials to integrate innovative therapies and address challenges early in the development process.
What services does bioaccess® offer for managing initial trials?
Bioaccess® offers comprehensive clinical trial management services, including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What insights can pilot study designs provide according to experts?
Expert opinions suggest that pilot study designs provide invaluable insights that can lead to adjustments in equipment design or reveal logistical challenges in trial management.
How do pilot studies impact the direction of larger investigations?
The results from pilot studies can determine whether to halt, modify, observe closely, or proceed with the main investigation based on the collected data.
What methodologies are used in pilot studies for medical devices?
Methodologies in pilot studies have evolved to include a blend of qualitative and quantitative approaches, such as structured interviews, surveys, and statistical assessments to gather comprehensive data.
How is sample size determined in pilot studies?
Sample size determination in pilot studies is grounded in feasibility objectives and can be complex, often utilizing a confidence interval approach to justify the sample sizes effectively.
What is the significance of aligning preliminary research objectives with pilot study designs?
Aligning the preliminary research objectives with pilot study designs enhances the trial process, leading to more successful outcomes in subsequent larger medical investigations.




