Introduction
In the complex landscape of medical device regulation, predicate devices serve as critical benchmarks for assessing the safety and effectiveness of new technologies. These legally marketed devices not only streamline the FDA's premarket notification process but also play a pivotal role in establishing substantial equivalence, which is essential for regulatory approval.
As the medical device market continues to evolve, understanding the nuances of predicate devices becomes paramount for manufacturers seeking to navigate the intricacies of compliance and market access.
This article delves into the significance of predicate devices, the processes for identifying them, and best practices for ensuring successful submissions, while also highlighting recent FDA guidance and the implications for device safety and efficacy.
With insights from industry experts and case studies, it aims to equip professionals with the knowledge necessary to enhance their regulatory strategies and foster greater consumer confidence in medical innovations.
Defining Predicate Devices: A Key Component in Medical Device Regulation
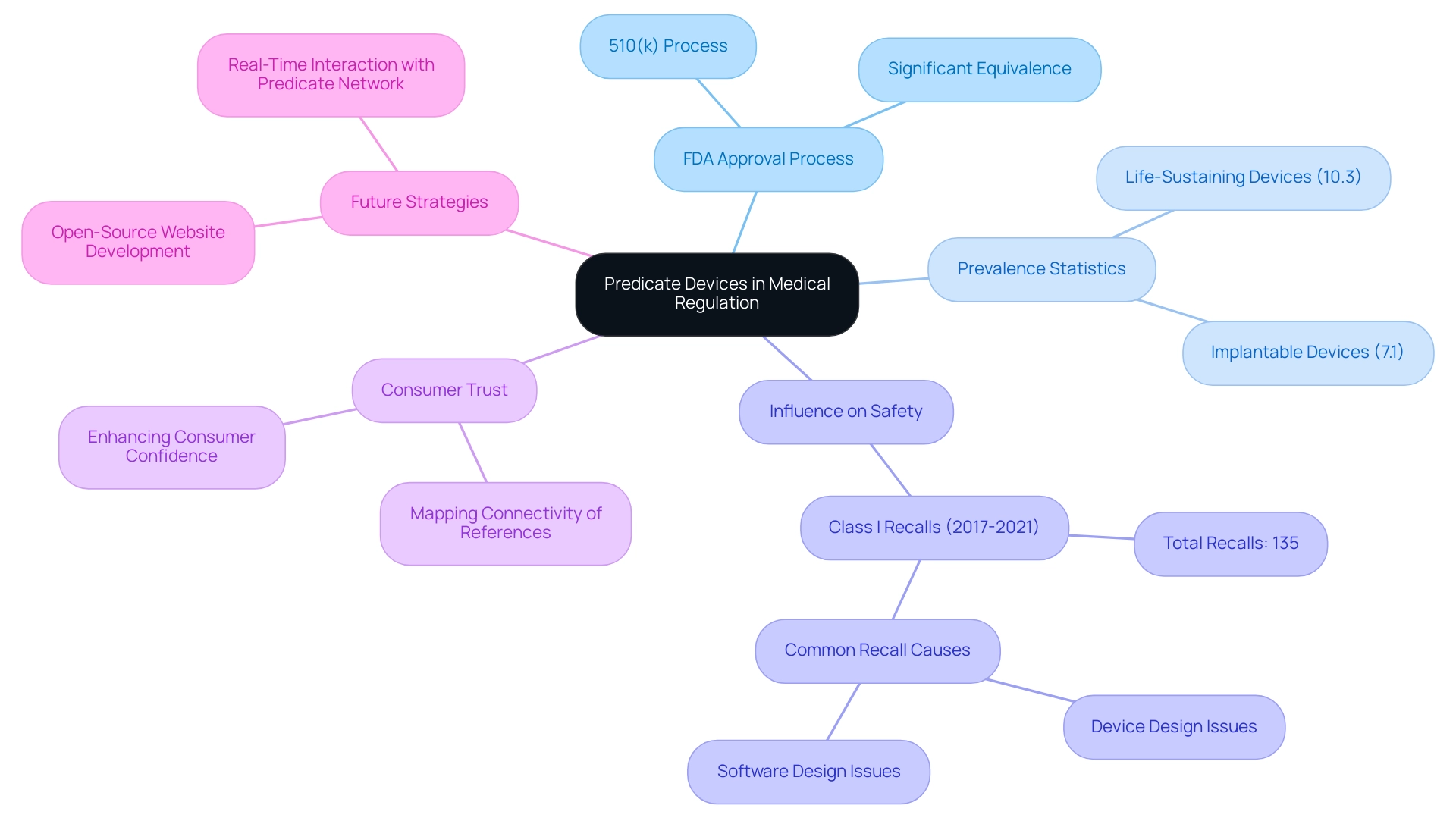
A predicate device meaning refers to a medical instrument that has been legally marketed and serves as a benchmark for assessing the safety and effectiveness of a new instrument within the framework of the FDA. The predicate device meaning plays a crucial role in establishing significant equivalence, which is essential for simplifying the approval process for new products. By permitting manufacturers to reference already approved products, these items facilitate the premarket notification process, commonly known as the 510(k) process.
This understanding is particularly crucial for professionals involved in product development, as it significantly influences FDA approval times and the overall regulatory strategy. According to recent findings, 16 life-sustaining or supporting instruments (10.3%) and 11 implantable tools (7.1%) were included in studies analyzing comparable products, underscoring their prevalence in the market. The implications of these statistics emphasize the essential role of such instruments in accelerating the approval procedure, ultimately improving market access for new medical technologies.
As Dhruv B. Pai pointed out,
In addition to providing a clearer comprehension of the risks and advantages of the 510(k) process, mapping the connectivity of various references could help enhance consumer trust in the medical products that are presently available in the market.
This emphasizes the significance of classification tools in promoting confidence and guaranteeing safety in medical equipment regulation. Additionally, upcoming strategies involve creating an open-source website for real-time interaction with the network, which could further improve comprehension and connectivity in this field.
Furthermore, a case study revealed a total of 135 entries of Class I recalls concerning 510(k) products from 2017 to 2021, highlighting the practical implications of reference items on product safety and regulation.
How to Identify Predicate Devices for Successful 510(k) Submissions
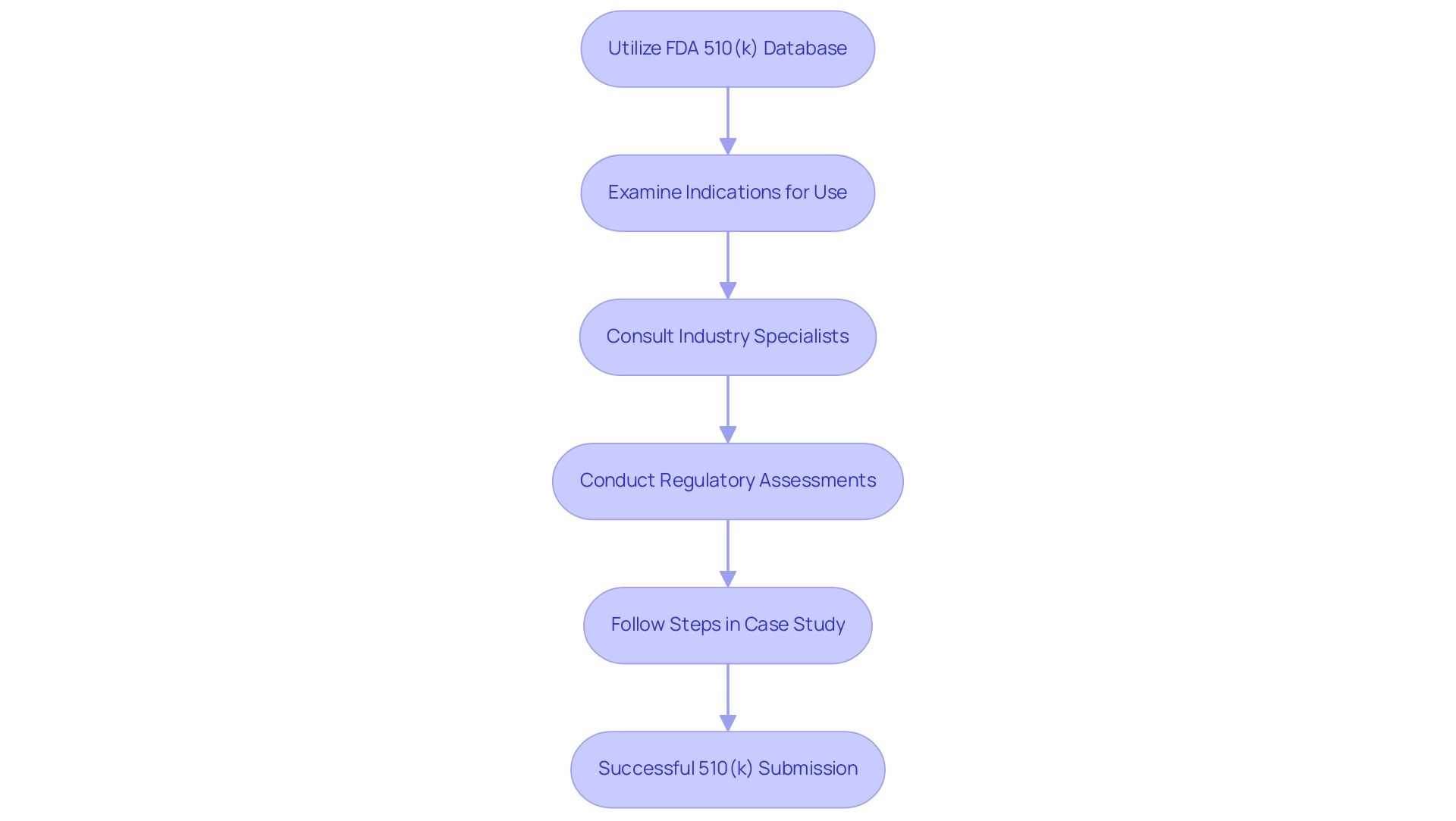
To effectively identify classification tools, one should start by utilizing the FDA’s 510(k) database, a comprehensive resource that enables searches based on product classification, intended use, and technological characteristics. With API updates occurring monthly, it is crucial to stay current with the latest information available in the database. Conducting a thorough examination of the indications for use and the specific design characteristics of potential comparison items is essential.
Interacting with industry specialists, like Ana Criado, a prominent affairs expert and CEO of Mahu Pharma, can provide valuable insights into the predicate device meaning of the most relevant devices. Ana's extensive experience as an external compliance consultant and professor of biomedical engineering positions her to provide critical guidance in this area. She employs methodologies such as detailed regulatory assessments and strategic compliance planning tailored to each client's needs.
Additionally, assistance is available for FDA registration and 510(k) submission, further supporting your efforts. A practical approach involves following the steps outlined in the case study titled 'Steps to Access 510(k) Data.' This procedure assists users in navigating the FDA's website, finding the searchable database, and entering specific search criteria such as product code or applicant name.
Remember, the closer a new device aligns with its predicate device meaning in terms of both technology and intended use, the higher the likelihood of a successful 510(k) submission. By adopting this meticulous approach, you can optimize your submission method and enhance compliance with evolving FDA regulations. Testimonials from clients who have successfully navigated the 510(k) process with Ana's guidance further underscore the effectiveness of her strategies.
Establishing Substantial Equivalence: Criteria and Best Practices
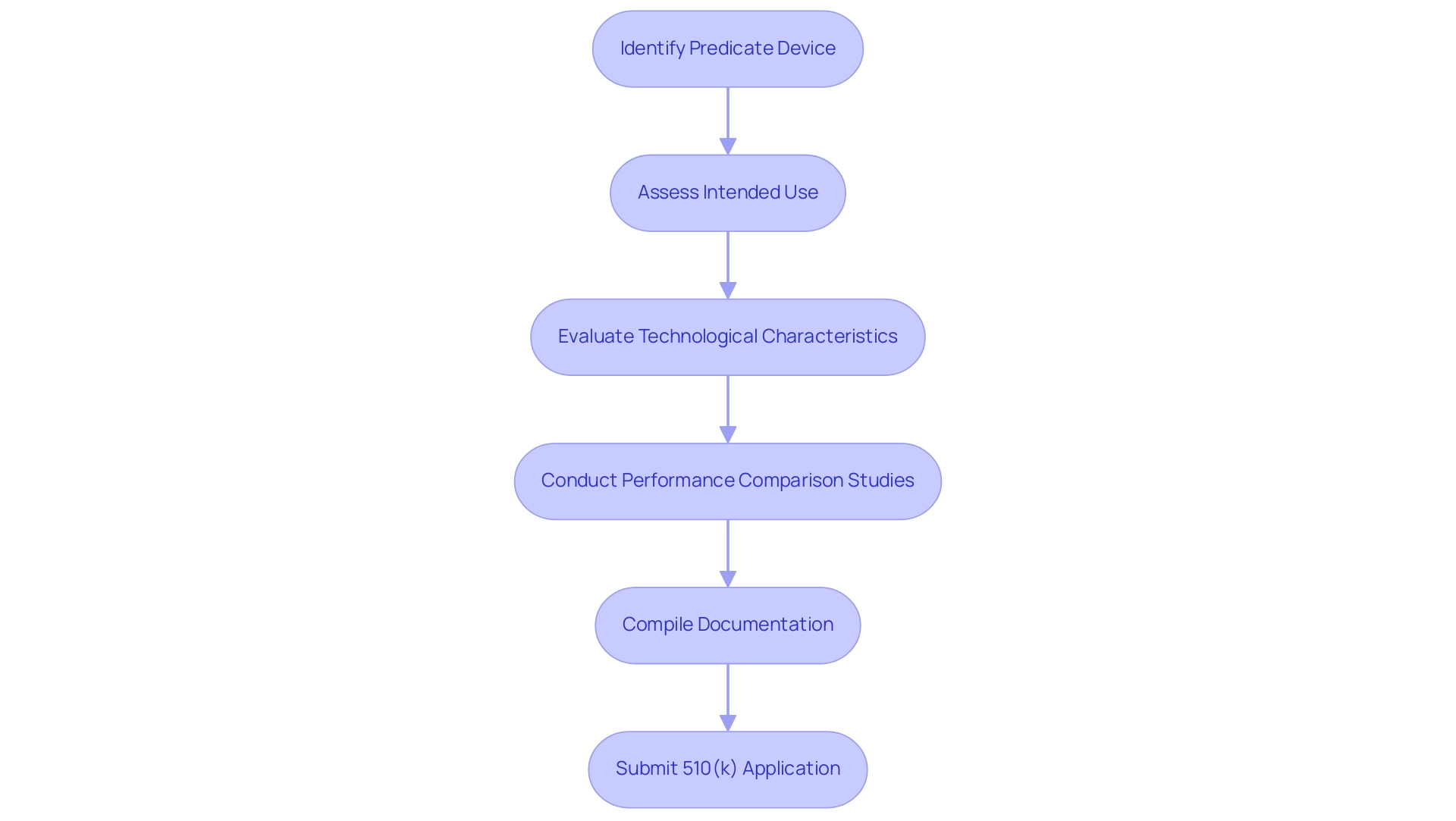
Establishing substantial equivalence is a critical requirement for new medical instruments seeking FDA approval, necessitating a comparison with a predicate device meaning based on intended use, technological characteristics, and performance outcomes. Key considerations during this evaluation encompass an assessment of materials, design, and potential risks associated with the instrument. Leveraging comprehensive clinical trial management services, including:
- Feasibility studies
- Trial setup
- Compliance reviews
- Import permits
- Project management
- Reporting on study status and adverse events
can significantly enhance this process.
Best practices for demonstrating substantial equivalence include conducting rigorous performance comparison studies and compiling comprehensive documentation that clearly addresses any differences, particularly in relation to predicate device meaning. A well-organized overview of information is crucial, demonstrating how the new product meets or exceeds the safety and effectiveness standards established by the original. Notably, statistics indicate that 75% of 510(k) submissions are rejected upon first review, with a staggering 85% of these rejections attributed to issues related to predicate device meaning during scientific appraisal.
This underscores the necessity for a robust risk mitigation strategy, as emphasized by industry experts:
To have a successful 510(k), you need to demonstrate a strong substantial equivalence and incorporate the predicate device meaning into your solid risk mitigation strategy. I think this is not to be neglected.
Furthermore, drawing parallels from a case study that compares a car and a truck reinforces the need to select a closely related predicate device meaning while compellingly justifying the equivalence.
The study references a total of 15 sources, of which only 8 are research articles, enhancing the credibility of the information presented. Moreover, with the knowledge of specialists such as Katherine Ruiz in regulatory affairs and Juan Cuya in clinical trials, who are proficient in techniques that enhance efficient project management and oversight, RegDesk streamlines global growth for medical products and IVD firms, emphasizing the wider ramifications of the significant equivalence procedure. By adhering to these best practices and being meticulous in the submission process, companies can significantly enhance their chances of a successful FDA review.
Navigating Recent FDA Guidance on Predicate Device Selection
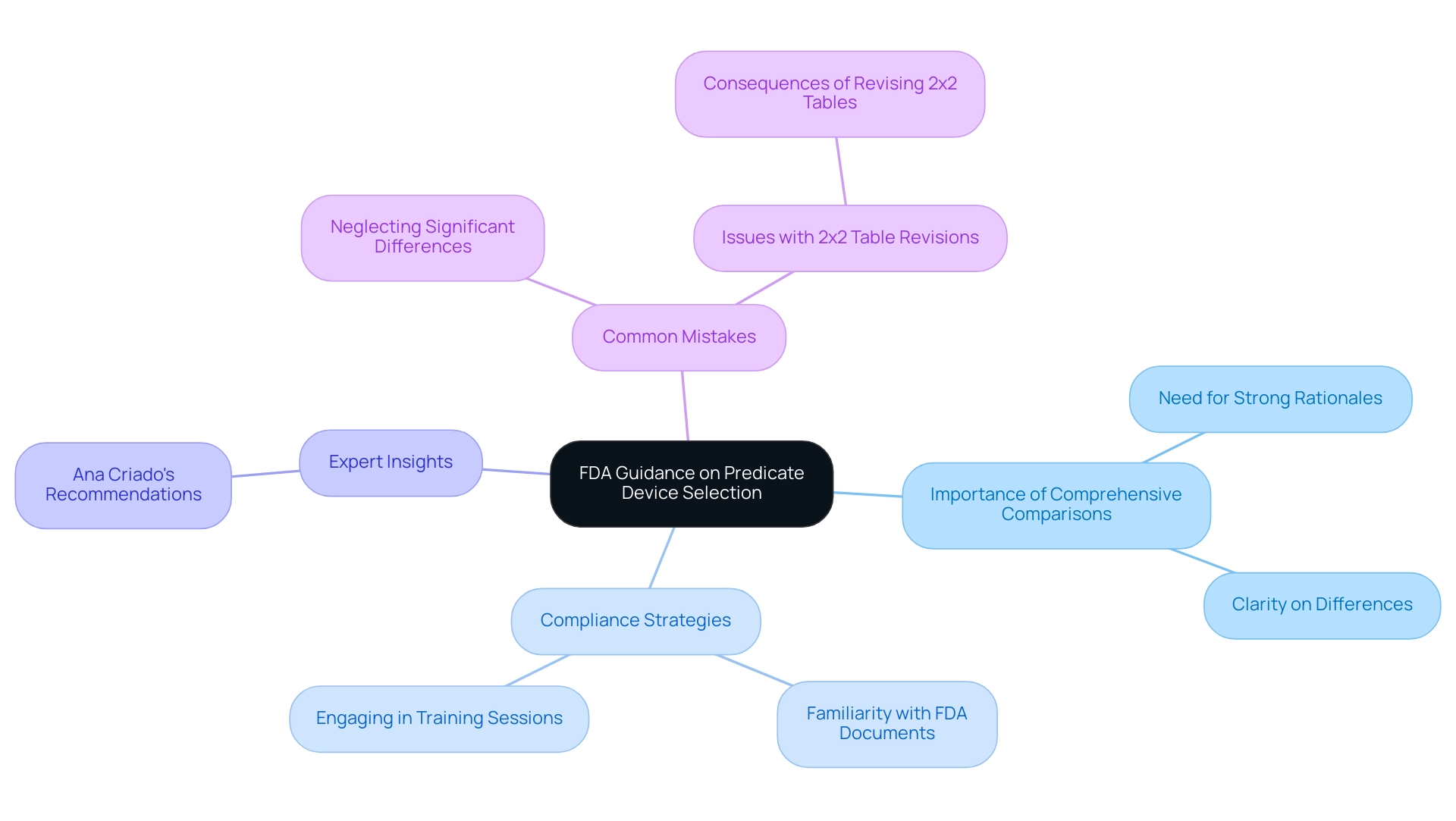
The most recent advice from the FDA emphasizes the essential importance of comprehensive comparisons of similar products, encouraging manufacturers to offer strong rationales for their choices. This focus on clarity is crucial, especially regarding any differences between the new gadget and its predecessor. Notably, public information about the predicate device meaning was missing for 17.3% of index devices, highlighting the need for greater clarity in this area.
Medical professionals navigating this compliance landscape should familiarize themselves with the latest FDA documents and recommendations, such as the FDA's guidance on 510(k) submissions, which detail the importance of demonstrating substantial equivalence. As emphasized by experts like Ana Criado, Director of Regulatory Affairs and a prominent figure in Colombian Regulatory Affairs, understanding these guidelines is vital for compliance. Ana, who has extensive experience with the INVIMA and is a consultant for major global companies, advocates for engaging in relevant training sessions to enhance understanding and ensure that submissions meet current expectations and best practices.
Furthermore, typical mistakes in comparison of products, such as neglecting significant differences in intended use or technological features, can result in unsuccessful submissions. The case study titled 'Consequences of Revising 2x2 Tables' illustrates the potential pitfalls in comparisons, reinforcing the necessity of adhering to good scientific practices. These proactive measures are essential for managing the complexities of selection of items.
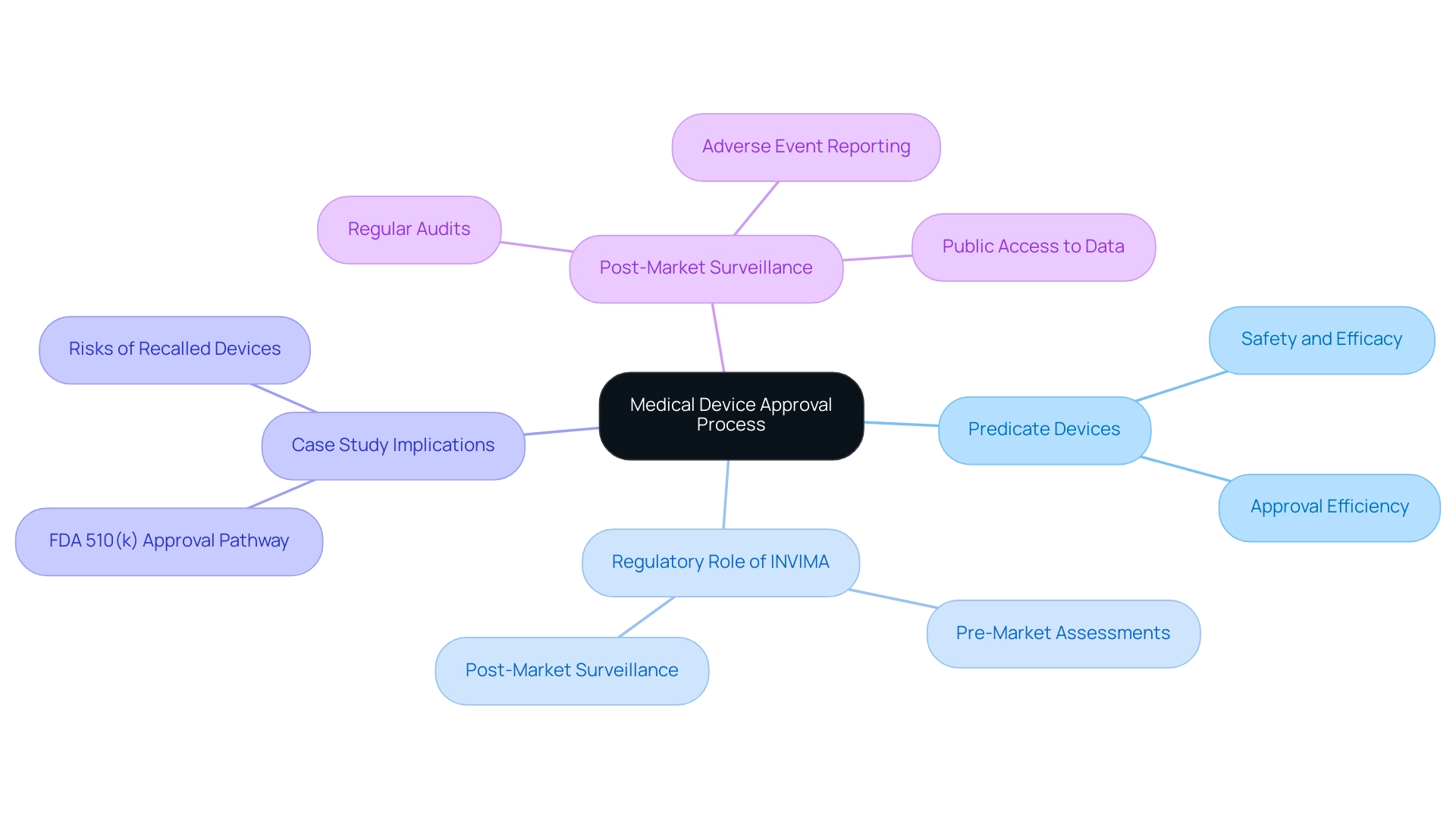
The Role of Predicate Devices in Medical Device Approval and Market Access
The predicate device meaning is essential to the medical apparatus approval procedure, serving as benchmarks that enable the evaluation of new items in terms of safety and efficacy. By referring to the predicate device meaning established by previous models, manufacturers can simplify the approval procedure, frequently leading to substantial decreases in both duration and resource use. This efficiency is crucial for product development teams, as it informs strategic planning and enables better allocation of resources.
In Colombia, the INVIMA (National Food and Drug Surveillance Institute) plays a pivotal role in this process, overseeing the regulatory framework for medical equipment. As a Level 4 health authority recognized by PAHO/WHO, INVIMA ensures that products meet safety and efficacy standards, providing medical approval for both imports and exports. INVIMA is specifically responsible for evaluating medical products through rigorous pre-market assessments and post-market surveillance, ensuring compliance with national and international standards.
A recent case study titled 'Evaluation of FDA 510(k) Approval Pathway' highlights the implications of using recalled reference products in the approval process, revealing that many new items approved through this pathway were based on references that had previously been subject to Class I recalls. This raises concerns about the safety and effectiveness of these new products, particularly given that the median number of units recalled for each Class I recall entry stood at 9,345. Such statistics highlight the potential dangers linked to depending on flawed assumptions, necessitating a careful approach to gadget approval.
As mentioned by MD Robert W. Yeh, the features of compliance submissions and the recalls of medical instruments necessitate careful scrutiny to protect patient safety. Therefore, understanding the dynamics of predicate device meaning can foster improved collaboration with regulatory bodies like INVIMA, facilitating smoother market access for new medical innovations in Colombia. Furthermore, robust post-market surveillance practices, such as regular audits and adverse event reporting, along with public access to data, are essential in ensuring ongoing device safety and quality, reinforcing the need for vigilance in monitoring the performance of devices after they reach the market.
Conclusion
Understanding the role of predicate devices is essential for navigating the complex landscape of medical device regulation. These benchmark devices not only facilitate the FDA's premarket notification process but also establish the substantial equivalence necessary for ensuring the safety and effectiveness of new technologies. By leveraging predicate devices, manufacturers can significantly streamline their regulatory pathways, enhancing their chances of successful submissions and ultimately fostering consumer confidence in medical innovations.
Identifying appropriate predicate devices involves a meticulous approach, utilizing resources such as the FDA’s 510(k) database and engaging with industry experts. The process requires careful consideration of the intended use and technological characteristics of both the new device and its predicate. Establishing substantial equivalence is critical, as it demands comprehensive documentation and rigorous performance comparisons to address any potential differences. The recent FDA guidance emphasizes the importance of transparency in these comparisons, underscoring the need for manufacturers to justify their selections thoroughly.
In conclusion, predicate devices are integral to both the approval process and market access for new medical technologies. As the landscape continues to evolve, staying informed about regulatory updates and best practices is paramount for manufacturers. By prioritizing thorough research and compliance, medical device professionals can enhance their regulatory strategies and contribute to the ongoing advancement of safe and effective medical innovations. The significance of predicate devices cannot be overstated; they are foundational to ensuring patient safety and fostering a robust medical device market.
Frequently Asked Questions
What is a predicate device?
A predicate device is a medical instrument that has been legally marketed and serves as a benchmark for assessing the safety and effectiveness of a new instrument within the FDA framework.
Why is the predicate device meaning important?
It plays a crucial role in establishing significant equivalence, which simplifies the approval process for new products by allowing manufacturers to reference already approved products during the premarket notification process, known as the 510(k) process.
How does the predicate device meaning influence FDA approval times?
Understanding predicate devices significantly influences FDA approval times and the overall regulatory strategy for professionals involved in product development.
What statistics highlight the prevalence of predicate devices in the market?
Recent findings show that 16 life-sustaining or supporting instruments (10.3%) and 11 implantable tools (7.1%) were included in studies analyzing comparable products.
How can mapping the connectivity of predicate devices enhance consumer trust?
It provides a clearer understanding of the risks and advantages of the 510(k) process, which can help enhance consumer trust in the medical products currently available in the market.
What future strategies are being proposed regarding predicate devices?
Upcoming strategies include creating an open-source website for real-time interaction with the network to improve comprehension and connectivity in the field.
What practical implications do predicate devices have on product safety and regulation?
A case study revealed a total of 135 entries of Class I recalls concerning 510(k) products from 2017 to 2021, highlighting the importance of reference items on product safety and regulation.
How can one effectively identify classification tools for predicate devices?
By utilizing the FDA’s 510(k) database, conducting thorough examinations of indications for use and design characteristics, and interacting with industry specialists for insights.
Who can provide valuable insights into predicate device meanings?
Industry specialists, such as Ana Criado, who is a prominent affairs expert, can provide critical guidance through detailed regulatory assessments and strategic compliance planning.
What steps can support FDA registration and 510(k) submission?
Following a practical approach outlined in case studies, such as the 'Steps to Access 510(k) Data,' can assist users in navigating the FDA's website and enhancing compliance with evolving regulations.




