Overview
Cost-efficient trial designs for Chile can be effectively implemented by:
- Leveraging local expertise
- Enhancing patient recruitment strategies
- Utilizing innovative study methodologies
This article underscores the significance of:
- Collaborations with local organizations
- Adaptive trial designs
These not only reduce operational costs but also elevate the overall efficiency and quality of clinical studies. Such factors position Chile as an attractive hub for Medtech research, fostering a conducive environment for groundbreaking advancements in the field.
Introduction
In the rapidly evolving field of medical technology, understanding the clinical trial landscape in Chile is crucial for stakeholders aiming to harness the country's potential for innovative research.
With a robust infrastructure supporting over 2,400 clinical studies and a regulatory framework that emphasizes international standards, Chile stands out as a promising hub for Medtech innovation.
The diverse demographics, particularly the significant proportion of the population under 14 years old, provide unique opportunities for targeted healthcare solutions.
As organizations increasingly seek to navigate the complexities of clinical trials, leveraging local expertise, implementing innovative trial designs, and maintaining a cycle of regular evaluations emerge as key strategies for success.
This article delves into the intricacies of conducting clinical trials in Chile, offering insights that can empower companies to optimize their research efforts and contribute to the advancement of healthcare in the region.
Understand the Chilean Clinical Trial Landscape
As of April 2025, the nation boasts a robust research study framework, with 2,482 studies currently documented. The regulatory environment is primarily overseen by the Instituto de Salud Pública (ISP), which ensures compliance with international standards, thereby enhancing the trustworthiness of medical studies conducted within the country. The typical approval duration for medical studies is notably efficient, ranging from 3 to 4 months, which is crucial for Medtech firms aiming to optimize their project timelines.
The diverse patient population, coupled with the increasing number of studies, creates significant opportunities for groundbreaking exploration. This demographic advantage is highlighted by the fact that approximately 30% of the population is under 14 years old, indicating a burgeoning market for pediatric and elderly healthcare solutions. A notable example of the nation’s research success is the first-in-human case of the SonoThrombectomy System, conducted at the Hospital DIPRECA, showcasing the country's capability in executing advanced medical studies.
Furthermore, recent investments in the clinical research sector, particularly in the Andean Region, suggest a positive trend, underscoring the nation’s potential as a hub for Medtech innovation. As Julio G. Martinez-Clark, CEO of bioaccess®, noted, "These advantages have attracted international medtech companies and fostered a culture of innovation within the healthcare sector."
Understanding local regulations and ethical considerations is vital for the successful execution of trials. The ongoing improvements in regulatory frameworks, such as those seen in Paraguay, serve as a regional model, promoting a culture of innovation and enticing international Medtech companies. By leveraging Paraguay's advancements, the nation can further refine its regulatory landscape. As the environment evolves, grasping these dynamics and the common challenges in navigating the research process will be essential for stakeholders looking to utilize cost-efficient trial designs in Chile's research ecosystem. Moreover, bioaccess® offers comprehensive services that encompass regulatory approval, site activation, subject recruitment, and data management, which are critical for enhancing the efficacy of studies. The impact of these initiatives extends beyond individual enterprises, contributing to local economies through job creation and healthcare advancements.
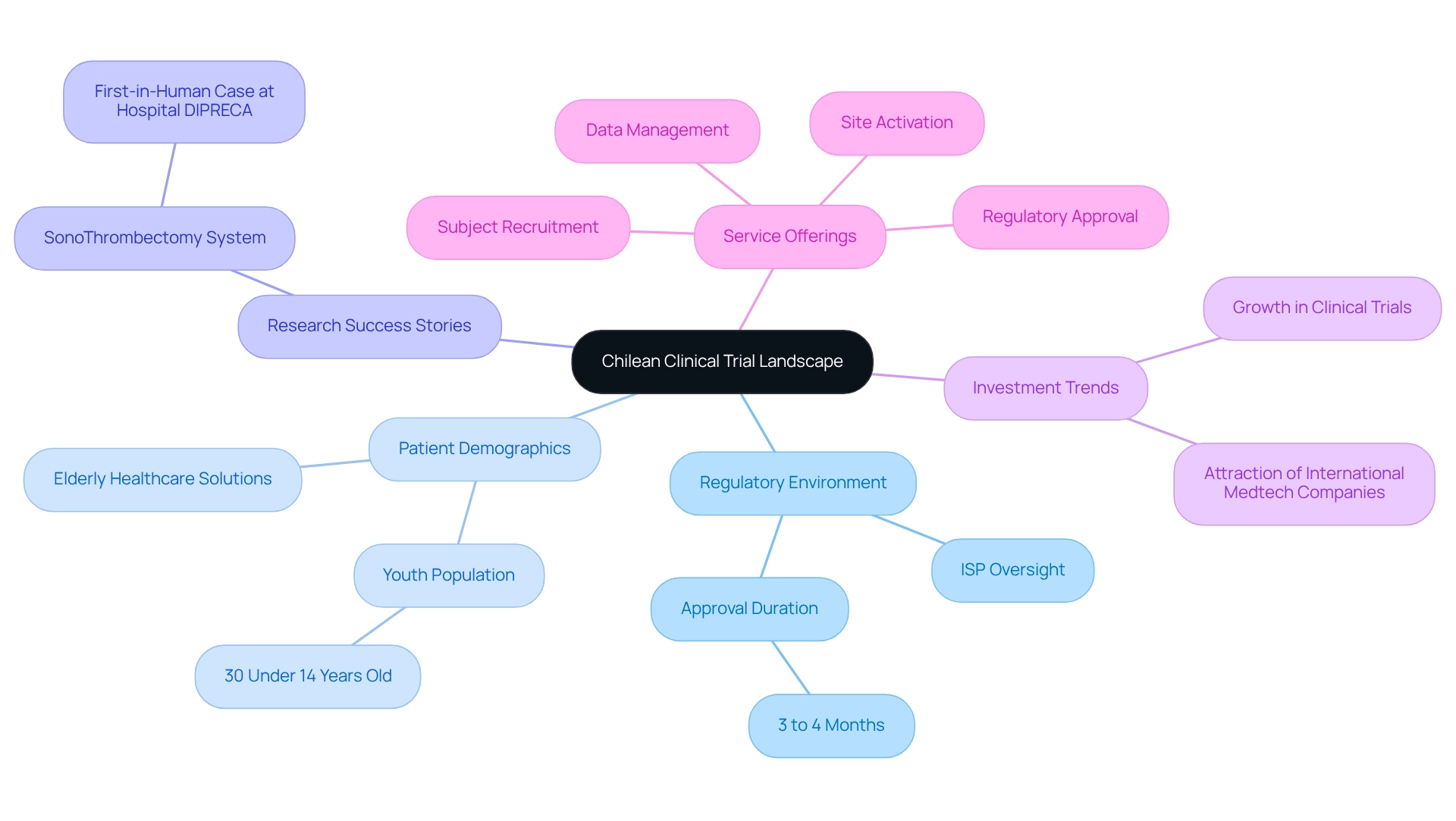
Leverage Local Expertise and Resources
Collaborating with local research organizations, universities, and healthcare providers is essential for developing cost-efficient trial designs in Chile. These partnerships provide crucial insights and resources that significantly enhance the efficiency of studies. They not only facilitate patient recruitment but also improve data quality and ensure adherence to local regulations. Engaging local investigators with a profound understanding of the patient population can lead to more effective recruitment strategies, as demonstrated by the success rates in recent studies. For instance, the NeuroStim study revealed that 70% of participants experienced notable decreases in depression symptoms, underscoring the positive impact of well-executed research.
Furthermore, leveraging local resources can substantially reduce operational expenses and improve logistics, rendering the overall study design more economical. A study published in the BMJ indicates that cost-efficient trial designs for Chile can be up to 30% less expensive than conducting medical studies in North America or Europe, highlighting the financial advantages of these collaborations. By fostering effective partnerships between universities and CROs, stakeholders can maximize their funding while enhancing the quality and pace of medical studies.
In Colombia, bioaccess™ has played a critical role in advancing medical device evaluations through a comprehensive suite of services, including feasibility studies, investigator selection, study setup, regulatory compliance, and project management. Their collaboration with the Colombian Minister of Health aims to position Barranquilla as a leading hub for medical studies in Latin America, further enhancing the region's appeal for health research.
Key Strategies for Leveraging Local Expertise and Resources:
- Engage Local Investigators: Utilize local expertise to enhance patient recruitment strategies.
- Collaborate with Universities: Partner with academic institutions to access resources and knowledge.
- Utilize Local Facilities: Take advantage of local infrastructure to reduce costs and improve logistics.
- Focus on Patient Experience: As James Merlino states, "In today’s competitive healthcare landscape, focusing on patient experience is essential for organizational success."
- Stay Informed on Healthcare Developments: Recognize the evolving role of Chile's healthcare system in shaping medical device development in Latin America.
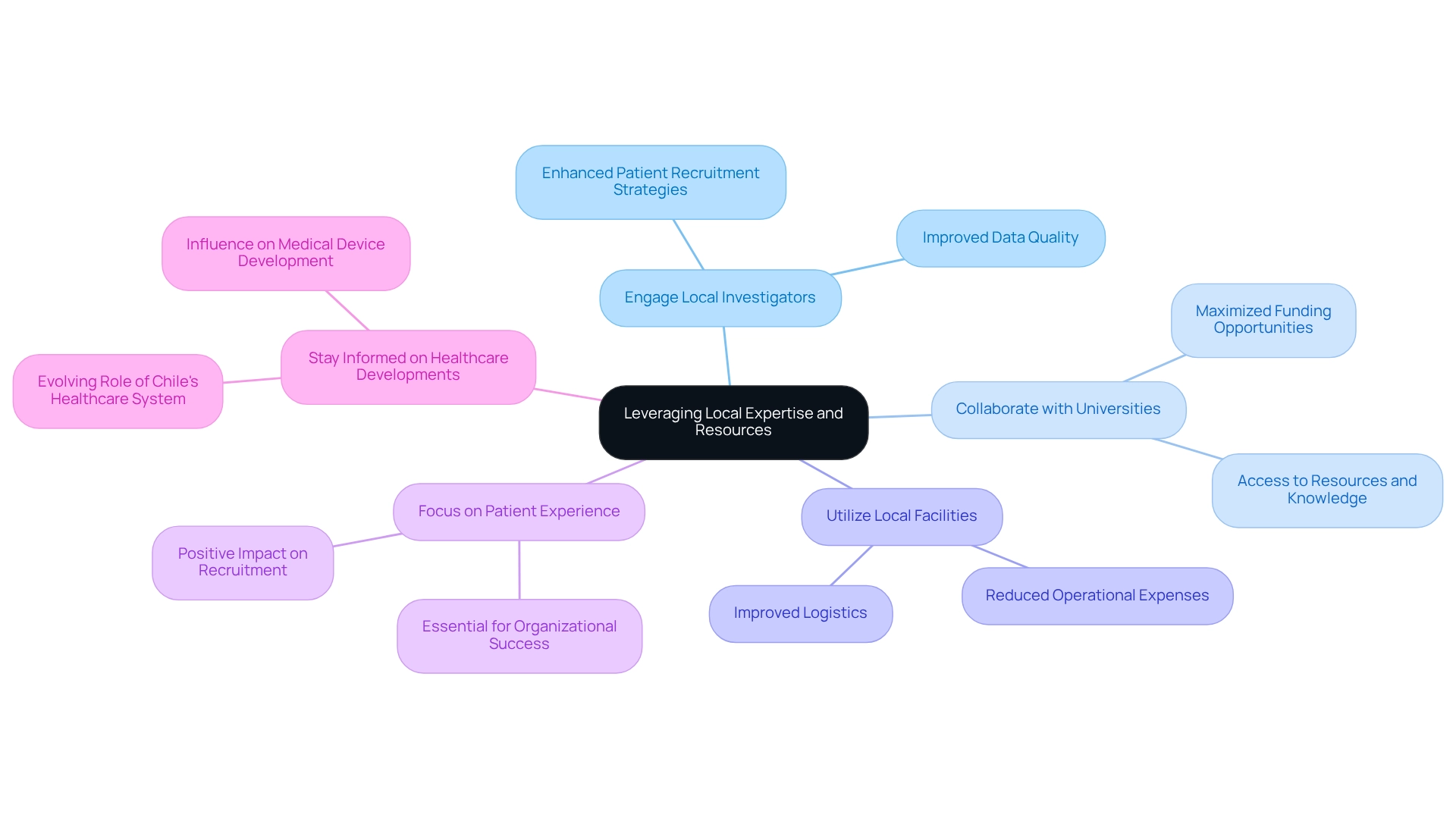
Implement Innovative Trial Design Strategies
Implementing innovative study design strategies, particularly cost-efficient trial designs for Chile, is crucial for enhancing the efficiency of clinical studies. These designs facilitate modifications based on interim results, empowering researchers to make informed decisions regarding patient enrollment and treatment protocols.
For example, the planned maximum sample size for the Giles et al. study involved 75 participants, illustrating how adaptive designs can optimize resource allocation. The application of Bayesian methods not only improves patient outcomes by focusing efforts on the most promising treatment options but also mitigates potential simulation bias. This concern is underscored in the FDA Draft Guidance, which indicates that simulation bias can occur when scenarios are selectively chosen to obscure the advantages of competing designs.
Furthermore, the integration of digital health technologies, such as remote monitoring and telemedicine, significantly enhances patient involvement and streamlines data collection, thereby accelerating the overall process. However, it is vital to consider the heterogeneity within patient populations across the interim stages of adaptive studies, as this can complicate the interpretation of results.
Practical challenges linked to adaptive designs must be meticulously managed to adhere to regulatory standards. Bioaccess's comprehensive research management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—equip Medtech companies to effectively navigate these complexities.
As the landscape of medical studies evolves, embracing cost-efficient trial designs for Chile will be essential for Medtech firms striving to enhance study execution and foster economic growth in regional markets.
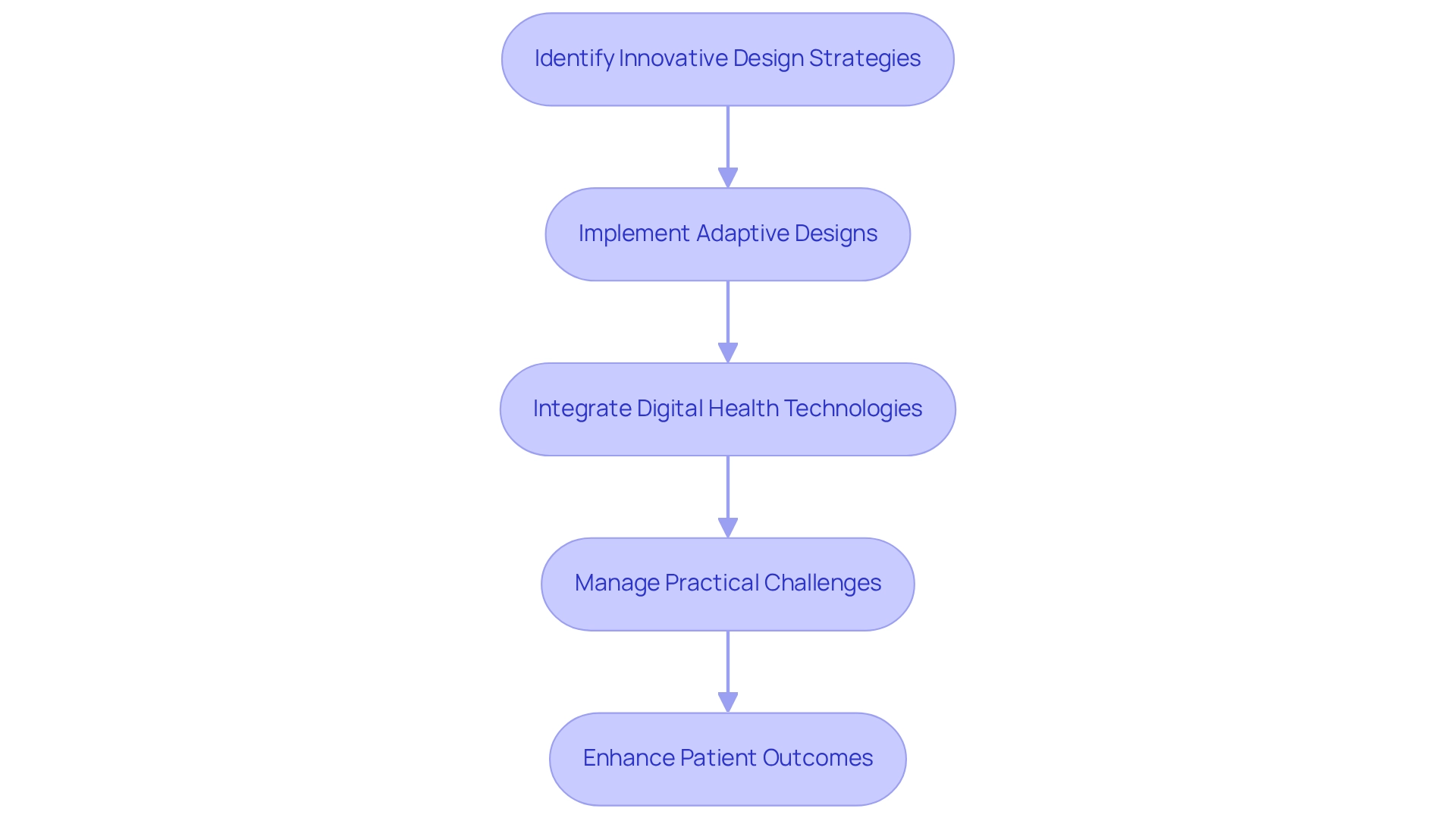
Evaluate and Adapt Trial Processes Regularly
Creating a robust framework for the regular assessment of experimental processes is essential for ensuring quality and compliance in clinical research, particularly within the dynamic landscape of Latin America. Conducting interim analyses allows for timely evaluations of a study's progress, facilitating necessary adjustments to protocols or recruitment strategies. For example, in the multicenter study comparing tenecteplase to alteplase for ischemic stroke, an adaptive sample size re-estimation was executed after 100 participants, ultimately increasing the sample size to 202. This modification was crucial in confirming that tenecteplase was non-inferior to alteplase, with event rates of 22% for tenecteplase compared to 10% for alteplase, highlighting the effectiveness of interim analyses in enhancing study outcomes.
Establishing feedback loops with the research team and stakeholders enables swift adjustments, ensuring that the study remains aligned with its objectives. As Sarah Lee noted, "Outlining the goals up front can significantly enhance the decision-making process, as it clearly defines the criteria that will drive study adaptations." Furthermore, leveraging data analytics tools can provide valuable insights into patient recruitment trends and data integrity, allowing for proactive management of potential challenges. Frequent assessments not only improve decision-making but also enhance the overall effectiveness of research studies, ultimately facilitating the rapid advancement of medical devices in the competitive Medtech environment.
Moreover, bioaccess® offers comprehensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
Their expertise in executing early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies ensures that studies are not only efficient but also aligned with regulatory excellence. The Thrombectomy for Stroke study in Brazil, which was halted early for efficacy after 174 participants completed their 90-day follow-up, exemplifies the significant benefits of timely evaluations in clinical research. Final results should also account for any preplanned or ad hoc analyses conducted during the trial to ensure a thorough evaluation process.
Conclusion
The clinical trial landscape in Chile presents a wealth of opportunities for Medtech innovation, driven by a robust regulatory framework and a diverse patient population. With over 2,400 clinical studies currently in progress, the country's efficient approval processes and adherence to international standards position it as a competitive hub for medical research. The unique demographics, particularly a significant number of children, create an ideal environment for developing targeted healthcare solutions, as evidenced by successful trials like the SonoThrombectomy System.
Collaboration with local expertise, including clinical research organizations and universities, is essential for maximizing the potential of clinical trials in Chile. These partnerships facilitate patient recruitment, improve data quality, and reduce operational costs, making trials more efficient and effective. By leveraging local resources and focusing on patient experience, stakeholders can enhance the overall success of their research endeavors.
Furthermore, the implementation of innovative trial designs, such as adaptive strategies and the incorporation of digital health technologies, can significantly optimize clinical trial processes. Regular evaluations and adaptations based on interim results are crucial for maintaining quality and compliance in an ever-evolving research landscape. The ability to make informed adjustments enhances patient outcomes and accelerates the advancement of medical devices.
In summary, Chile's clinical trial environment offers a promising landscape for Medtech companies willing to engage with local resources and innovate in trial design. By understanding and navigating the complexities of this dynamic field, stakeholders can contribute to the growth of healthcare solutions that benefit both local populations and the broader medical community. Embracing these strategies will ultimately lead to groundbreaking advancements in healthcare and economic growth within the region.
Frequently Asked Questions
How many research studies are currently documented in the nation as of April 2025?
As of April 2025, there are 2,482 research studies documented in the nation.
Who oversees the regulatory environment for medical studies in the country?
The regulatory environment is primarily overseen by the Instituto de Salud Pública (ISP), which ensures compliance with international standards.
What is the typical approval duration for medical studies in the nation?
The typical approval duration for medical studies is efficient, ranging from 3 to 4 months.
What demographic advantage does the nation have for medical studies?
Approximately 30% of the population is under 14 years old, indicating a burgeoning market for pediatric and elderly healthcare solutions.
Can you provide an example of a successful medical study conducted in the nation?
A notable example is the first-in-human case of the SonoThrombectomy System, conducted at the Hospital DIPRECA.
What recent trends are observed in the clinical research sector in the Andean Region?
Recent investments in the clinical research sector suggest a positive trend, highlighting the nation’s potential as a hub for Medtech innovation.
Why is understanding local regulations and ethical considerations important for clinical trials?
Understanding local regulations and ethical considerations is vital for the successful execution of trials and navigating the research process effectively.
How can Paraguay's advancements in regulatory frameworks benefit the nation?
Paraguay's advancements can serve as a regional model, promoting a culture of innovation and enticing international Medtech companies to the nation.
What services does bioaccess® offer to enhance the efficacy of studies?
Bioaccess® offers comprehensive services that include regulatory approval, site activation, subject recruitment, and data management.
What impact do these initiatives have on local economies?
These initiatives contribute to local economies through job creation and advancements in healthcare.




