Overview
Creating scalable trial designs is essential for enhancing the efficiency and adaptability of clinical research. This approach allows studies to be modified based on real-time data and participant feedback, addressing the dynamic nature of clinical trials.
By incorporating flexibility, efficiency, and patient-centricity into trial designs, researchers can effectively respond to emerging data. This optimization not only improves resource allocation but also enhances participant engagement, ultimately leading to more successful outcomes in the Medtech sector.
The importance of these designs cannot be overstated; they represent a pivotal shift in how clinical research can evolve to meet the challenges of today’s healthcare landscape.
Introduction
In the rapidly evolving landscape of clinical research, scalable trial designs are emerging as a game-changer, offering the flexibility and efficiency needed to navigate the complexities of modern medical studies. These innovative methodologies empower researchers to adapt trial parameters in real-time, responding dynamically to participant feedback and emerging data. As the demand for faster and more effective clinical outcomes grows, understanding the nuances of scalable designs becomes essential for success.
This article delves into the key components of scalable trial designs, their operational considerations, and the future trends shaping their implementation, providing valuable insights for researchers aiming to enhance the efficacy and responsiveness of clinical trials in an increasingly competitive Medtech environment.
Understanding Scalable Trial Designs in Clinical Research
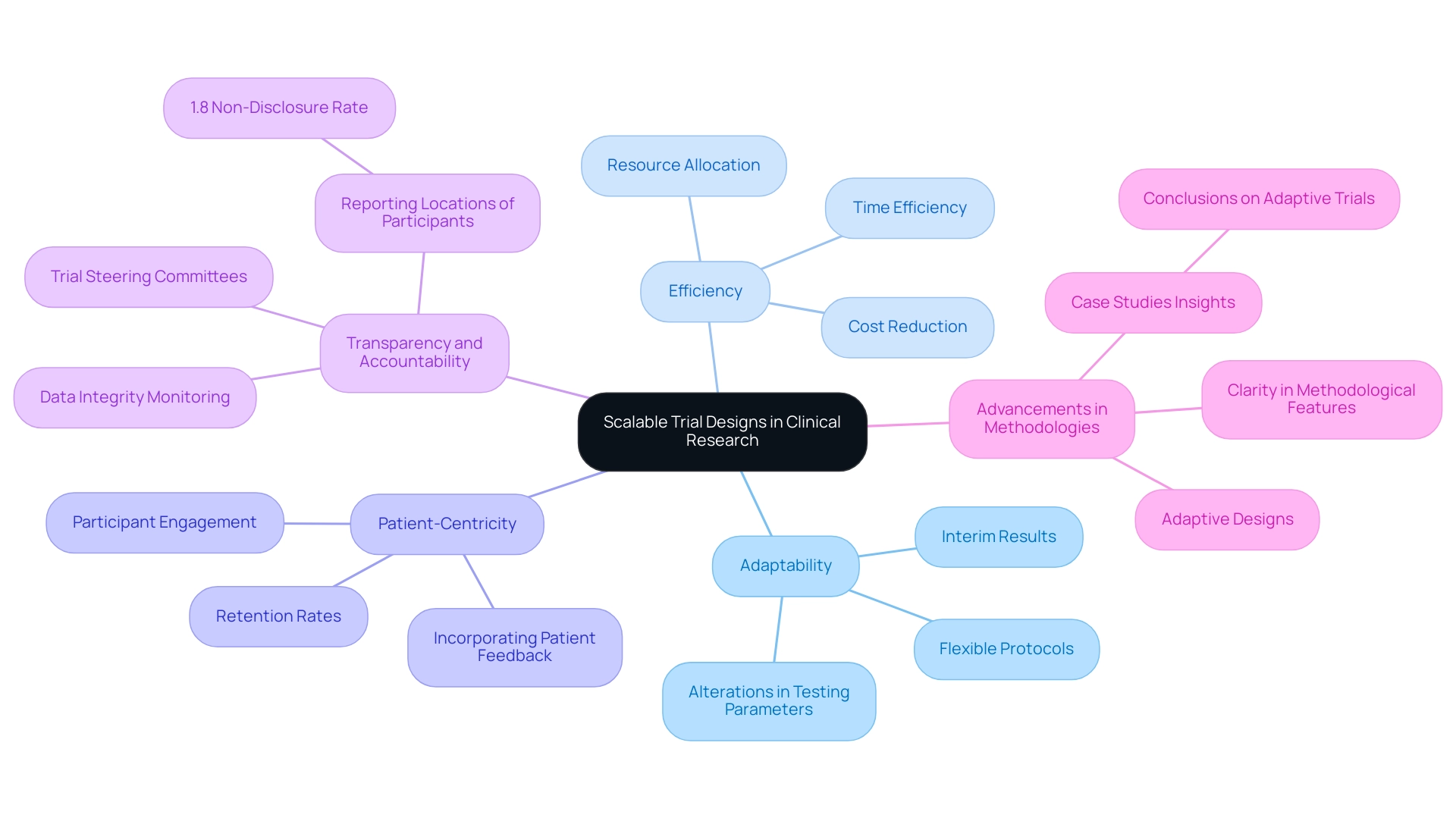
Creating scalable trial designs signifies groundbreaking techniques that empower studies to be modified in size and scope based on real-time data and participant feedback. These plans are essential in contemporary clinical research, enabling researchers to allocate resources efficiently and adapt to unforeseen challenges. Key elements of scalable trial designs include adaptability, efficiency, and patient-centricity.
First, creating scalable trial designs allows for adaptability by enabling alterations in testing parameters, such as sample size and treatment protocols, driven by interim results. This adaptability is crucial for responding to emerging data and optimizing results. As Professor Salaheddin M Mahmud emphasizes, flexibility is a cornerstone of effective clinical study formulation, ensuring that studies can evolve in response to new insights. At bioaccess®, our extensive experience in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, and Post-Market Clinical Follow-Up Studies (PMCF) positions us to implement these adaptable approaches effectively.
Second, efficiency plays a critical role. By enhancing resource allocation, adaptable structures can significantly reduce the expenses and time typically associated with conventional testing approaches. This efficiency becomes increasingly vital as the demand for rapid results grows within the Medtech sector. With over 20 years of expertise, bioaccess® enhances study setups and compliance evaluations, ensuring our clients achieve their goals swiftly and effectively.
Furthermore, patient-centricity is paramount. Incorporating patient feedback into scalable designs not only enhances participant engagement but also improves retention rates. This focus on the patient experience is becoming a cornerstone of successful medical studies. Our approach at bioaccess® underscores the significance of patient participation, which is essential for the success of research studies in Latin America.
Recent advancements in scalable research methodologies have underscored the advantages of flexibility. A review on adaptive studies highlights their role in efficiently informing optimal treatment practices, emphasizing the necessity for clarity regarding methodological, operational, and ethical features to foster familiarity and uptake in clinical research. This aligns with the findings from the case analysis titled "Conclusions on Adaptive Trials," which stresses the importance of adaptive approaches in enhancing test effectiveness.
Moreover, recent statistics reveal that only 1.8% of studies fail to disclose the location of study participants, indicating a growing trend towards transparency and accountability in research frameworks. This statistic underscores the increasing focus on transparent reporting and governance, with steering committees supervising studies to ensure safety and proper conduct. As research leaders advocate for the significance of adaptability, creating scalable trial designs is poised to enhance the efficiency and responsiveness of investigations in the Medtech sector.
By adopting these principles, researchers can significantly elevate the overall success of their trials, bridging the gap between innovative Medtech companies and the potential for undertaking research projects in Latin America. This ultimately aids in job creation, economic growth, and healthcare advancement in the region.
The Role of Innovation in Clinical Trial Design
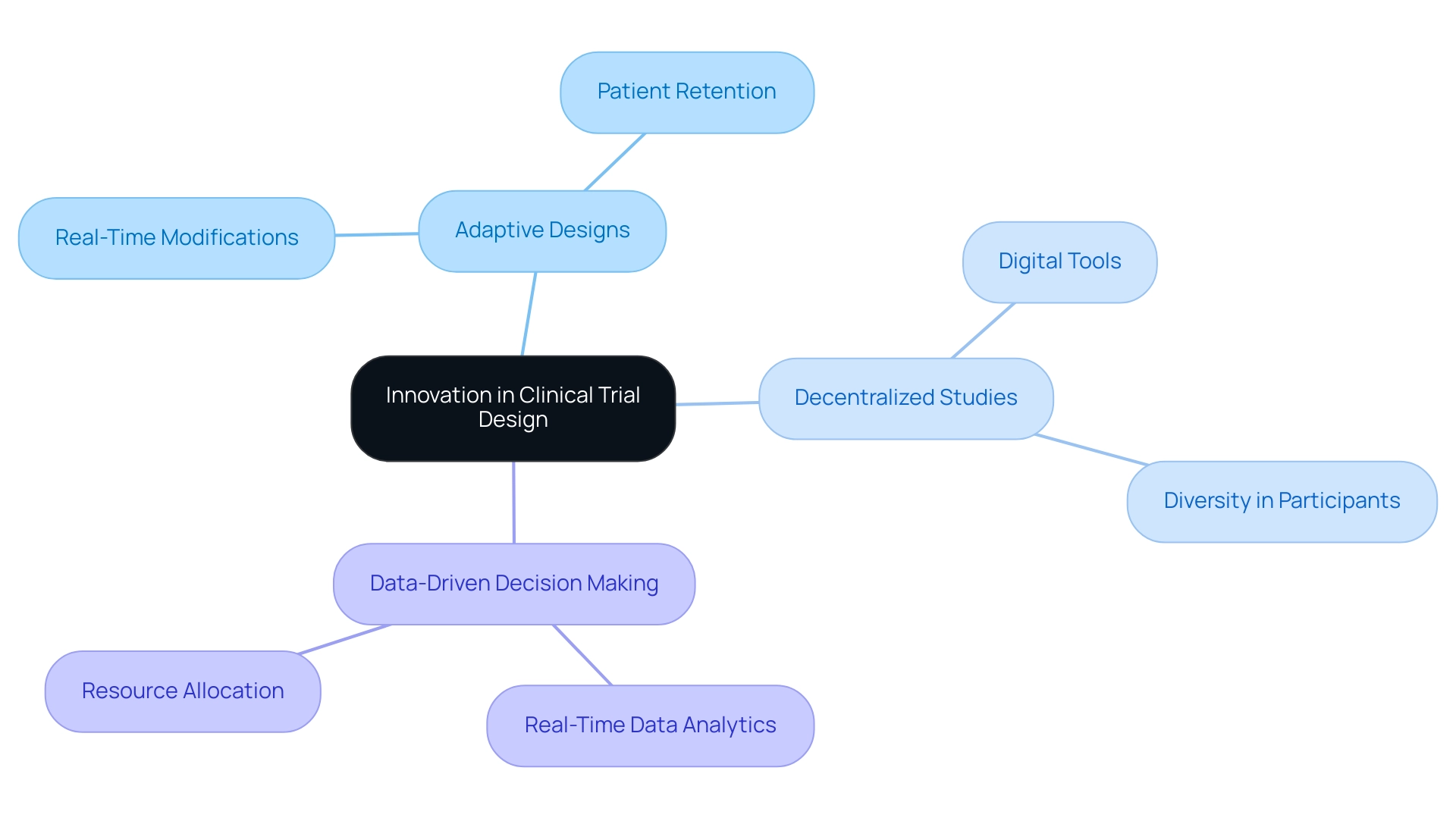
Creating scalable trial designs is crucial for improving efficiency and effectiveness in clinical study formulation, particularly in the rapidly evolving Medtech environment. Key components of this innovation include adaptive designs, decentralized studies, and data-driven decision-making.
Adaptive Designs: These methodologies facilitate real-time modifications based on interim results, allowing researchers to dynamically adjust strategies. This flexibility significantly influences outcome results, as recent research indicates that adaptive approaches can shorten duration and enhance patient retention rates. bioaccess® exemplifies this approach, leveraging over 20 years of expertise to implement adaptive designs in various studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
Decentralized Studies: The integration of digital tools and remote monitoring capabilities has transformed conventional study frameworks. Decentralized studies not only enhance patient access but also promote diversity in participant demographics, aligning with the latest FDA guidance urging sponsors to enroll more patients from underrepresented groups. This approach is pivotal for ensuring that research findings are applicable to a broader population. bioaccess® is at the forefront of this movement, partnering with Caribbean Health Group to establish Barranquilla as a premier research location in Latin America, supported by Colombia's Minister of Health.
Data-Driven Decision Making: The utilization of real-time data analytics empowers researchers to make informed adjustments throughout the experimental process. By analyzing data as it is collected, medical teams can identify trends and potential issues early, leading to improved outcomes and more efficient resource allocation. For instance, eliminating just one 20-minute task per visit across a large-scale experiment can save thousands of hours of work, enabling Clinical Research Associates (CRAs) to focus on essential research aspects. The collaboration between GlobalCare Clinical Trials and bioaccess™ has demonstrated this efficiency, achieving over a 50% decrease in recruitment time and 95% retention rates.
The expansion of global multi-regional research (MRCT) further underscores the significance of these innovations, allowing researchers to conduct investigations across diverse populations and regulatory environments, thereby enhancing the generalizability of findings. Moreover, the emphasis on MDR solutions highlights the current challenges in research methodology and data handling, emphasizing the need for creative strategies to simplify procedures and enhance data accuracy.
The impact of these advancements is profound, as they not only streamline research processes but also elevate the overall quality of medical investigations. As the sector continues to advance, adopting these innovative methods will be critical for researchers aiming to meet the demands of contemporary studies and improve patient outcomes. Recent trends indicate a growing reliance on adaptive approaches, with industry leaders advocating for the development of scalable trial designs to maximize the effectiveness of clinical studies in the Medtech sector.
The case of bioaccess® illustrates this shift, as the organization employs innovative testing methods to facilitate the rapid advancement of medical devices in Latin America, bridging the gap between innovation and market entry. bioaccess® specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), utilizing a customized approach tailored to the unique requirements of each study.
Exploring Different Types of Clinical Trial Designs
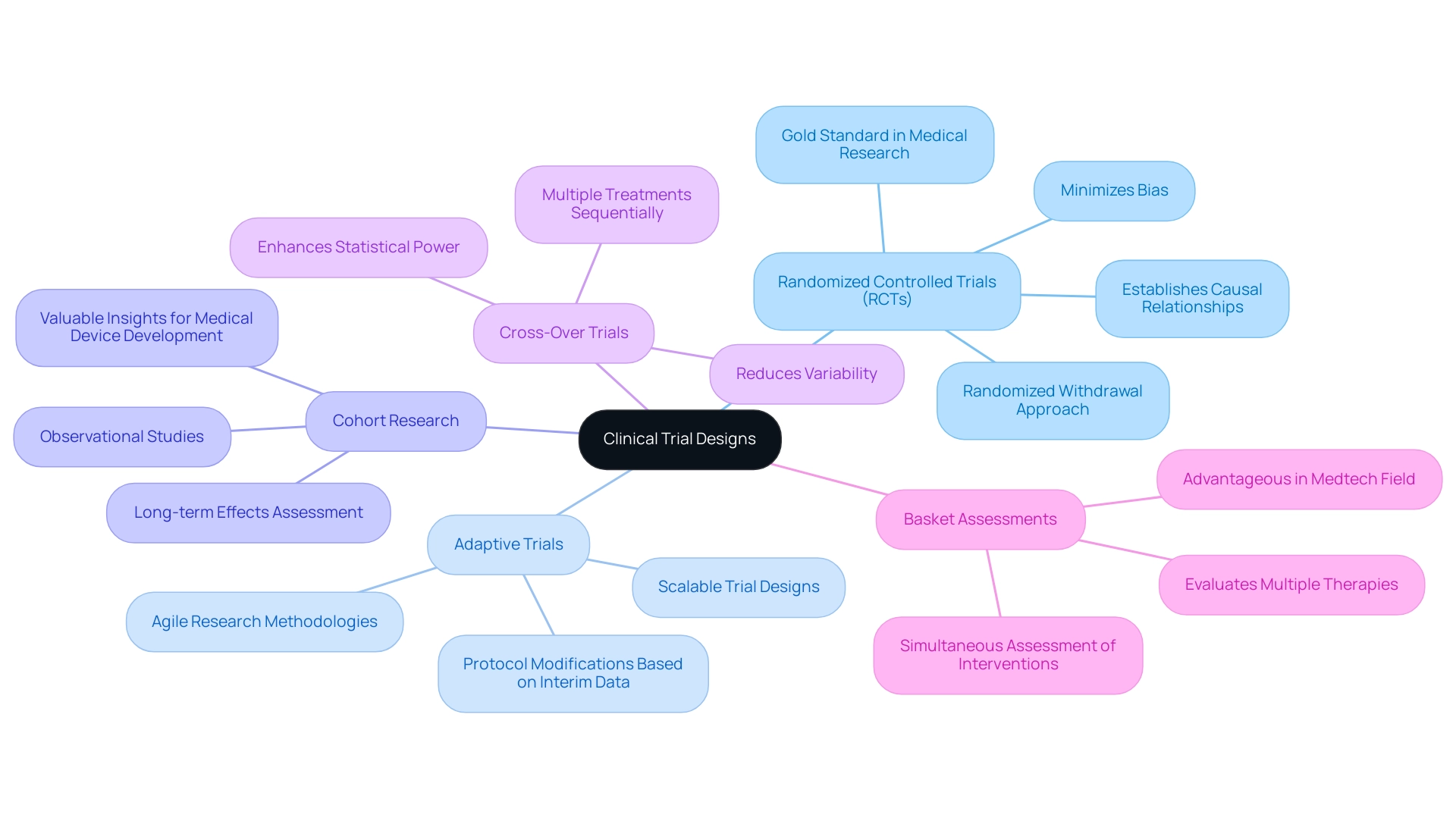
Clinical research frameworks encompass a variety of methodologies, each tailored to specific research objectives and contexts. Grasping these designs is essential for developing scalable trial designs, optimizing study outcomes, and ensuring regulatory compliance, particularly in Latin America where bioaccess® operates. The primary types of clinical trial designs include:
-
Randomized Controlled Trials (RCTs): Often regarded as the gold standard in medical research, RCTs effectively minimize bias by randomly assigning participants to treatment or control groups. This framework is especially adept at establishing causal relationships and evaluating the efficacy of interventions. Recent trends indicate that RCTs continue to dominate clinical research, with a significant percentage of new studies employing this methodology to ensure robust data collection. Additionally, the randomized withdrawal approach addresses ethical concerns regarding placebo use, reinforcing its relevance in study frameworks.
-
Adaptive Trials: These innovative designs allow for modifications to the protocol based on interim data analysis. This flexibility aids in creating scalable trial designs, enabling researchers to adjust sample sizes, treatment regimens, or endpoints in response to emerging results. Adaptive studies are increasingly preferred in the Medtech sector, where rapid advancements demand agile research methodologies. bioaccess® prioritizes the development of scalable trial designs while managing adaptive trials to ensure alignment with evolving data and regulatory requirements.
-
Cohort Research: As observational studies, cohort research monitors participants over time to assess outcomes based on specific exposures. They are particularly beneficial for understanding long-term effects and can yield valuable insights when RCTs are impractical due to ethical or logistical barriers. bioaccess® leverages its extensive experience to conduct comprehensive cohort analyses that produce meaningful data for medical device development.
-
Cross-Over Trials: In this design, participants receive multiple treatments sequentially, facilitating direct comparisons within the same individual. This approach can enhance statistical power and reduce variability, making it a compelling choice for research with limited sample sizes. bioaccess® employs cross-over studies to maximize the efficiency of clinical research, particularly during early-feasibility and pilot phases.
-
Basket Assessments: These assessments evaluate multiple therapies targeting a single disease type, enabling simultaneous assessment of various interventions. This structure is particularly advantageous in the Medtech field, where diverse treatment options may be explored for a common condition. bioaccess® excels at orchestrating basket studies, facilitating comprehensive evaluations of innovative medical devices.
Creating scalable trial designs reveals distinct strengths and weaknesses inherent in each experimental design. For example, while RCTs offer high-quality evidence, they can be resource-intensive and time-consuming. In contrast, adaptive studies provide flexibility but may introduce complexities in data interpretation.
A recent internal pilot study illustrates the value of a phased approach, where the initial pilot phase continues until the requisite sample size for the definitive phase is attained, thus optimizing resource utilization and minimizing patient wastage.
As we look to 2025, the landscape of clinical studies continues to evolve, with randomized controlled experiments remaining a cornerstone of clinical research. R.A. Fisher aptly remarked, "To call in the statistician after the experiment is done may be no more than asking him to perform a post-mortem examination - he may be able to say what the experiment dies of." This underscores the importance of meticulous experimentation from inception to conclusion.
By judiciously selecting the appropriate experimental framework, researchers can enhance the credibility of their results and contribute to the advancement of medical technologies, which is vital for developing scalable trial designs. With over 20 years of experience in the Medtech sector, bioaccess® is well-equipped to assist researchers in navigating these complex methodologies, ensuring compliance with local regulations, including those established by INVIMA, and achieving successful outcomes in studies.
Operational Considerations for Scalable Trial Designs
Implementing scalable trial designs necessitates a thorough examination of several operational factors that can significantly influence the success of clinical research initiatives:
-
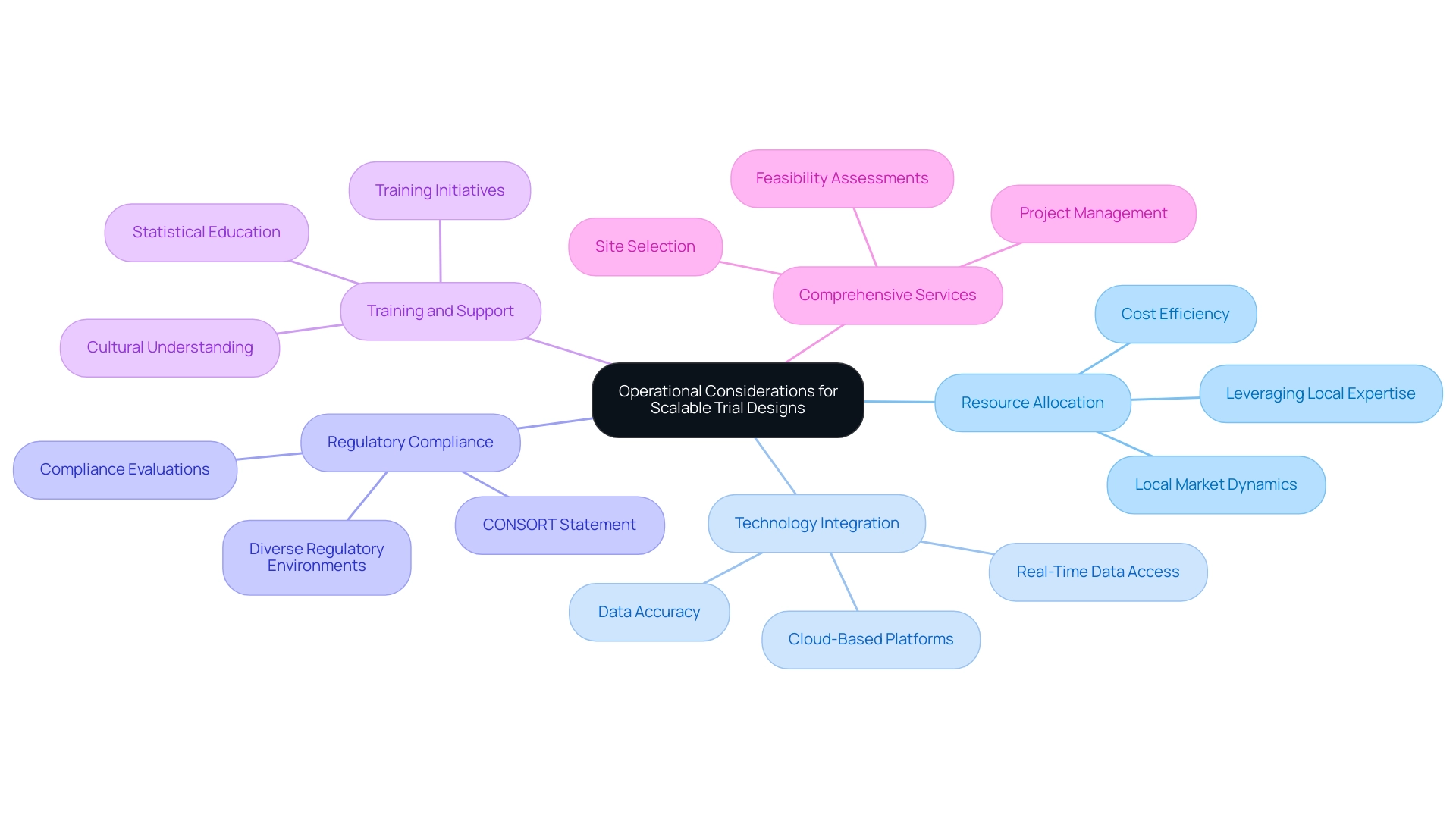
Resource Allocation: Effective management of resources, including personnel and funding, is paramount for achieving scalability. Research shows that optimal resource allocation can decrease expenses by up to 30%, facilitating a more efficient use of available funds. In the context of Latin America, understanding local market dynamics and leveraging local expertise can further enhance resource allocation strategies.
-
Technology Integration: The adoption of advanced digital tools for data collection and monitoring not only streamlines processes but also enhances data accuracy. For instance, integrating cloud-based platforms can facilitate real-time data access and improve collaboration among research teams, ultimately leading to more informed decision-making. Companies such as bioaccess® are leading the way in employing these technologies to improve research efficiency in Latin America, guaranteeing that studies are performed with the most advanced tools accessible.
-
Regulatory Compliance: Following regulatory guidelines is crucial for preserving the integrity of research studies. The CONSORT Statement, which includes a 22-item checklist and a flow diagram, serves as a valuable resource for ensuring that randomized clinical studies are reported comprehensively and transparently. In Latin America, navigating the diverse regulatory environments across countries is crucial, and organizations like bioaccess® provide expertise in compliance evaluations and setup to facilitate this process.
-
Training and Support: Offering extensive training for personnel involved in the study is vital for enhancing execution and participant engagement. As Scott R. Evans, Ph.D., from Harvard University points out, "It is also important that clinicians educate themselves about common statistical issues and concerns when conducting or participating in clinical studies." Research indicates that well-trained staff can improve recruitment rates and decrease dropout rates, thereby boosting the overall effectiveness of the study. In the Latin American context, local training initiatives can also foster better understanding and adherence to cultural nuances, further enhancing outcomes.
-
Comprehensive Services: bioaccess® provides a variety of services essential for successful research studies, including feasibility assessments, site selection, project management, and compliance evaluations. These services are intended to tackle the distinct challenges of performing research in Latin America, ensuring that studies are not only compliant but also strategically positioned for success.
By addressing these operational considerations, researchers can significantly enhance the likelihood of successful study outcomes, which is essential for creating scalable trial designs that pave the way for innovative medical advancements and ensure that healthcare practices are grounded in reliable evidence. The difficulties encountered in research studies, as emphasized in the case analysis titled 'The Role of Research Studies in Medical Advancements,' highlight the importance of these operational elements in attaining effective and efficient medical research.
Addressing Common Challenges in Clinical Trial Recruitment and Management
Recruitment and management challenges present significant obstacles in research studies, directly impacting their success rates. Addressing these challenges is crucial for enhancing participant engagement and ensuring efficient workflows.
Participant Awareness: A considerable number of potential participants remain unaware of ongoing clinical studies. To combat this, implementing targeted outreach strategies—such as community engagement initiatives and digital marketing campaigns—can significantly enhance awareness and interest in participation.
Eligibility Criteria: Strict eligibility requirements often restrict recruitment efforts. By assessing and possibly expanding these criteria, researchers can incorporate a more varied participant pool, which is essential for the generalizability of study results. This approach not only increases recruitment numbers but also enriches the data collected.
Logistical Barriers: Geographic and transportation challenges can deter participation, particularly in underserved areas. Adopting decentralized study designs, which allow for remote monitoring and local site visits, can effectively mitigate these logistical issues, making participation more accessible. For example, GlobalCare Clinical Studies has partnered with bioaccess™ to broaden its research ambulatory services in Colombia, utilizing bioaccess™'s extensive presence to improve accessibility and support for participants.
Retention Strategies: Maintaining participant engagement throughout the study is vital for success. Regular communication, personalized support, and flexible scheduling can significantly enhance retention rates. For instance, GlobalCare Clinical Trials has achieved over a 95% retention rate through effective participant engagement strategies, demonstrating the importance of consistent follow-ups and feedback mechanisms in enhancing participant satisfaction and reducing dropout rates.
As clinical study sponsors prioritize addressing site capacity challenges in 2025, standardizing technology across studies will be essential. Bree Burks, Vice President of Site Strategy at Veeva, observes that "as sponsors reconsider their site engagement approaches in 2025, they will emphasize consistent site technology and standardization among sponsors for all studies." This standardization can streamline processes, reduce administrative burdens, and ultimately improve recruitment and retention outcomes.
Experts emphasize that while global studies offer numerous advantages, the complexities of varying regulations and cultural differences present substantial challenges. The case study titled "Challenges of Differing Regulations in Global Trials" illustrates these complexities, highlighting how sponsors must navigate diverse regulatory environments, which can significantly impact patient enrollment and retention. By proactively tackling these recruitment and management challenges, researchers can improve their processes, thereby creating scalable trial designs that result in more successful and efficient studies.
Furthermore, offering additional options for patient onboarding and visitations will enhance enrollment and retention of diverse populations, aligning with the strategies discussed.
Additionally, bioaccess™ provides extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting. These capabilities are essential in addressing the challenges discussed and ensuring the success of clinical studies.
Step-by-Step Guide to Creating Scalable Trial Designs
Developing scalable experimental frameworks necessitates a systematic method that encompasses several essential steps:
- Define Objectives: Begin by articulating the study's goals and the specific research questions it seeks to address. This clarity is essential for guiding all subsequent decisions.
- Select Design Type: Choose a test format that aligns with the defined objectives and the characteristics of the target population. The layout should facilitate robust data collection and analysis, leveraging bioaccess's expertise in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
- Develop Protocol: Formulate a comprehensive protocol detailing eligibility criteria, treatment regimens, and data collection methodologies. A well-structured protocol serves as the backbone of the study, ensuring consistency and compliance, particularly in the context of regulatory requirements set forth by INVIMA, Colombia's National Food and Drug Surveillance Institute, which oversees medical device classification and compliance as a Level 4 health authority by PAHO/WHO.
- Incorporate Flexibility: Integrate adaptive elements into the framework, allowing for modifications based on interim results. This flexibility can enhance the experiment's responsiveness to emerging data and improve overall outcomes.
- Engage Stakeholders: Actively involve key stakeholders—such as regulatory bodies, patient advocacy groups, and clinical experts—early in the planning process. Their insights can significantly influence the project's feasibility and acceptance, ensuring alignment with regulatory expectations and patient needs.
- Pilot Testing: Conduct preliminary trials to evaluate the practicality of the plan and pinpoint possible challenges prior to large-scale implementation. Statistics indicate that pilot testing can reveal critical insights, such as the ceiling effect observed in goal attainment scores, which may impact the expected value and variance of outcomes. Significantly, Cohen's effect size for correlation studies indicates that even minor effects can be substantial in study formulation, underscoring the importance of statistical rigor.
Moreover, the introduction of an Experiment quality score by Statsig emphasizes the necessity of following best practices in study execution, thereby enhancing experimentation rigor. The suggested statistical framework for assessing experiments with a GAS endpoint further emphasizes current progress in planning and data analysis, rendering the content timely and pertinent for 2025.
Expert insights, such as those from Brian Fox regarding sequential experiment structure, reinforce the significance of interim monitoring and sample size re-evaluation in study frameworks. By following these steps and incorporating these insights, researchers can focus on creating scalable trial designs that satisfy regulatory standards and improve the chances of successful outcomes. The incorporation of best practices and stakeholder involvement throughout the process is essential for managing the intricacies of research in 2025, especially with the assistance of bioaccess's extensive management services, supported by over 20 years of experience in Medtech and a personalized strategy designed to fulfill client needs.
Ensuring Regulatory Compliance in Scalable Trial Designs
Ensuring regulatory compliance in scalable trial designs is paramount and involves several critical considerations:
-
Understand Regulations: Familiarizing oneself with both local and international rules governing clinical studies is essential. This foundational knowledge underpins compliant study designs and operations.
-
Documentation: Maintaining meticulous documentation of all testing processes is crucial. This encompasses protocols, consent forms, and data management practices. Effective documentation not only supports regulatory compliance but also enhances the integrity of the research data.
-
Ethics Committees: Submitting research designs to ethics committees for review and approval is vital to uphold ethical standards. The role of ethics boards in medical research is significant; they serve as protectors of participant welfare and bolster public confidence in research studies. As we approach 2025, the importance of ethics committee approval will only grow, particularly with the anticipated implementation of the FDA's Single IRB Requirement. The FDA encourages sponsors to develop Diversity Action Plans that outline clear objectives for enrolling participants from diverse age, gender, racial, and ethnic backgrounds, emphasizing the necessity for inclusive clinical studies.
-
Comprehensive training for all staff involved in the study is essential. This ensures that everyone understands compliance requirements and the ethical implications of their roles, which is critical for maintaining high standards throughout the process.
-
Monitoring and Auditing: Implementing regular monitoring and auditing processes is necessary to ensure ongoing compliance throughout the study. These practices help identify potential issues early, allowing for timely corrective actions.
At bioaccess®, our service capabilities include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. We employ specific methodologies such as risk-based monitoring and creating scalable trial designs to improve efficiency and compliance. We prioritize regulatory compliance to protect participant welfare and enhance the credibility and reliability of research studies.
Clinical studies are essential for assessing novel therapies to ensure their safety and effectiveness before they are made available to the public. They involve volunteers who contribute to medical science and the development of new therapies. As highlighted in the case study titled 'The Role of Clinical Studies in Medical Progress,' research studies remain a cornerstone of healthcare advancement, addressing significant challenges and facilitating groundbreaking discoveries that enhance patient care worldwide.
By prioritizing regulatory compliance, researchers not only safeguard participant welfare but also enhance the credibility and reliability of their studies. As Samruddhi Yardi aptly stated, "Through the dedication of volunteers and the commitment of researchers, medical studies stand as a beacon of hope for a healthier, more resilient future, where medical knowledge continues to expand, and the quality of care continually improves for individuals worldwide." This commitment to ethical and regulatory standards is essential for advancing medical knowledge and improving patient care globally.
Furthermore, bioaccess® is dedicated to ensuring information security and client trust through robust data protection measures. For any queries or concerns regarding data protection, clients can reach out to our Grievance/Data Protection Officer at info@bioaccessla.com, ensuring transparency and compliance.
Future Trends in Scalable Clinical Trial Designs
The future of scalable clinical trial designs is being significantly influenced by several key trends that promise to reshape the landscape of clinical research in 2025 and beyond.
-
Artificial Intelligence: The integration of AI technologies is revolutionizing study designs by optimizing processes such as patient recruitment and data analysis. For instance, IDx Technologies has partnered with bioaccess™ to identify Latin American ophthalmology centers for AI-based disease detection collaboration, showcasing AI's ability to streamline operations. Success stories emphasize AI's ability to decrease participant recruitment duration by more than 50% and attain retention rates surpassing 95%, resulting in quicker and more effective studies. In 2025, we expect to see a broader adoption of methodologies leveraging human cells and tissues for drug discovery and development, further enhancing the role of AI in clinical research.
-
Real-World Evidence: The incorporation of real-world data into study designs is gaining traction, enhancing the relevance and applicability of findings. This approach allows researchers to capture patient experiences and outcomes in diverse settings, ultimately leading to more meaningful insights. As Max Baumann, Head of Execution at Treehill Partners, noted, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
-
Decentralization: The trend towards decentralized studies is expected to continue, making participation more accessible and inclusive. This shift not only expands the participant pool but also accommodates diverse patient populations, which is crucial for the generalizability of study results. 'GlobalCare Clinical Studies' partnership with bioaccess™ to enhance clinical study ambulatory services in Colombia exemplifies this trend, as it supports global pharmaceutical clients in meeting their clinical study patient in-home needs.
-
Patient Engagement: An increased emphasis on patient-centered approaches is fostering greater involvement and retention. By prioritizing the needs and preferences of patients, researchers can foster a more collaborative environment that enhances the overall research experience.
-
Adaptive Approaches: The use of adaptive study frameworks is increasing, enabling more responsive and flexible research methodologies. This approach enables researchers to make real-time adjustments based on interim results, optimizing the process's trajectory towards achieving both approval-enabling endpoints and commercial success. The obstacles encountered in research design underscore the need for inventive frameworks that address real-world implementation issues and patient experiences, resulting in a transition towards practical study designs that offer more significant insights into medical interventions across varied global contexts.
Moreover, the extensive research management services provided by bioaccess™, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting, are vital in supporting these trends. The recent FDA approval of Adaptimmune's Tecelra, a cancer cell therapy for solid tumors, underscores the importance of these trends in advancing medical technologies and highlights bioaccess™'s role in facilitating such advancements through its robust service offerings.
By embracing these emerging trends, clinical researchers can significantly enhance the scalability and effectiveness of their trials, which is essential for creating scalable trial designs that ultimately lead to improved patient outcomes and a more efficient pathway to bringing innovative medical technologies to market.
Conclusion
Scalable trial designs are revolutionizing the field of clinical research, providing the necessary flexibility and efficiency to meet the evolving demands of modern medical studies. Essential components such as adaptability, efficiency, and a patient-centric approach are critical for navigating the complexities inherent in clinical trials. By harnessing real-time data, researchers can optimize trial parameters, minimize costs, and enhance participant engagement, ultimately leading to more successful outcomes.
As the Medtech sector continues to progress, the significance of innovative methodologies like adaptive and decentralized trial designs becomes increasingly clear. These strategies not only streamline processes but also ensure that clinical findings remain relevant and applicable to diverse populations. Furthermore, addressing operational considerations—such as resource allocation, regulatory compliance, and staff training—will further bolster the success of scalable trial designs.
Looking to the future, the integration of artificial intelligence and real-world evidence is poised to further influence the landscape of clinical trials. By embracing these trends and prioritizing patient engagement, researchers can enhance the scalability and effectiveness of their studies, paving the way for significant breakthroughs in medical technology. Ultimately, a steadfast commitment to innovative, flexible, and responsive trial designs will not only improve patient outcomes but also propel the advancement of healthcare on a global scale.
Frequently Asked Questions
What are scalable trial designs in clinical research?
Scalable trial designs are innovative methodologies that allow studies to be modified in size and scope based on real-time data and participant feedback, enhancing adaptability, efficiency, and patient-centricity.
How do scalable trial designs enhance adaptability in clinical studies?
They enable researchers to alter testing parameters, such as sample size and treatment protocols, driven by interim results, allowing for timely responses to emerging data and optimization of study outcomes.
Why is efficiency important in scalable trial designs?
Efficiency allows for better resource allocation, significantly reducing the time and expenses associated with traditional testing methods, which is increasingly vital in the Medtech sector that demands rapid results.
What role does patient-centricity play in scalable trial designs?
Incorporating patient feedback into the design enhances participant engagement and retention rates, making the patient experience a cornerstone of successful medical studies.
What recent advancements have been made in scalable research methodologies?
Recent advancements highlight the benefits of flexibility in adaptive studies, which improve treatment practices and emphasize the importance of clear methodological, operational, and ethical features for effective clinical research.
What is the significance of transparency in clinical studies?
A growing trend towards transparency and accountability in research frameworks is indicated by recent statistics showing only 1.8% of studies fail to disclose participant locations, highlighting the need for oversight and safety in studies.
How can adopting scalable trial designs impact the success of clinical trials?
By implementing scalable trial designs, researchers can improve the overall success of their trials, facilitating innovative Medtech companies to conduct research in regions like Latin America, ultimately aiding in job creation and healthcare advancement.
What methodologies are included in scalable trial designs?
Key methodologies include adaptive designs, decentralized studies, and data-driven decision-making, each contributing to the flexibility and efficiency of clinical research.
How do decentralized studies benefit clinical trials?
Decentralized studies enhance patient access and promote diversity in participant demographics, aligning with FDA guidance to enroll underrepresented groups, which ensures broader applicability of research findings.
What impact does data-driven decision-making have on clinical research?
Utilizing real-time data analytics allows researchers to make informed adjustments during trials, leading to improved outcomes and more efficient resource allocation, significantly reducing recruitment time and enhancing retention rates.

