Overview
The primary focus of the article is the effective design of clinical trials for medical devices in Bolivia. It delineates essential steps in the clinical trial process, including:
- Preclinical studies
- Regulatory requirements
- Effective recruitment strategies
The article underscores the necessity of adhering to local regulations while advocating for the inclusion of diverse participant populations. This approach is vital for enhancing the reliability and applicability of study results, ultimately ensuring that clinical trials are both compliant and representative.
Introduction
Navigating the intricate landscape of clinical trials for medical devices is essential for ensuring the safety and efficacy of innovative technologies. Each phase, from foundational preclinical studies to rigorous post-market surveillance, plays a critical role in the development process.
In Bolivia, understanding regulatory requirements is paramount; compliance with local laws can significantly impact the success of a trial. Furthermore, designing a robust clinical trial protocol and implementing effective recruitment strategies are vital components that can determine the outcome of these studies.
This article delves into the essential aspects of clinical trials for medical devices, offering insights into processes, regulatory frameworks, and strategies that can enhance participant recruitment and overall trial success.
Understand the Basics of Clinical Trials for Medical Devices
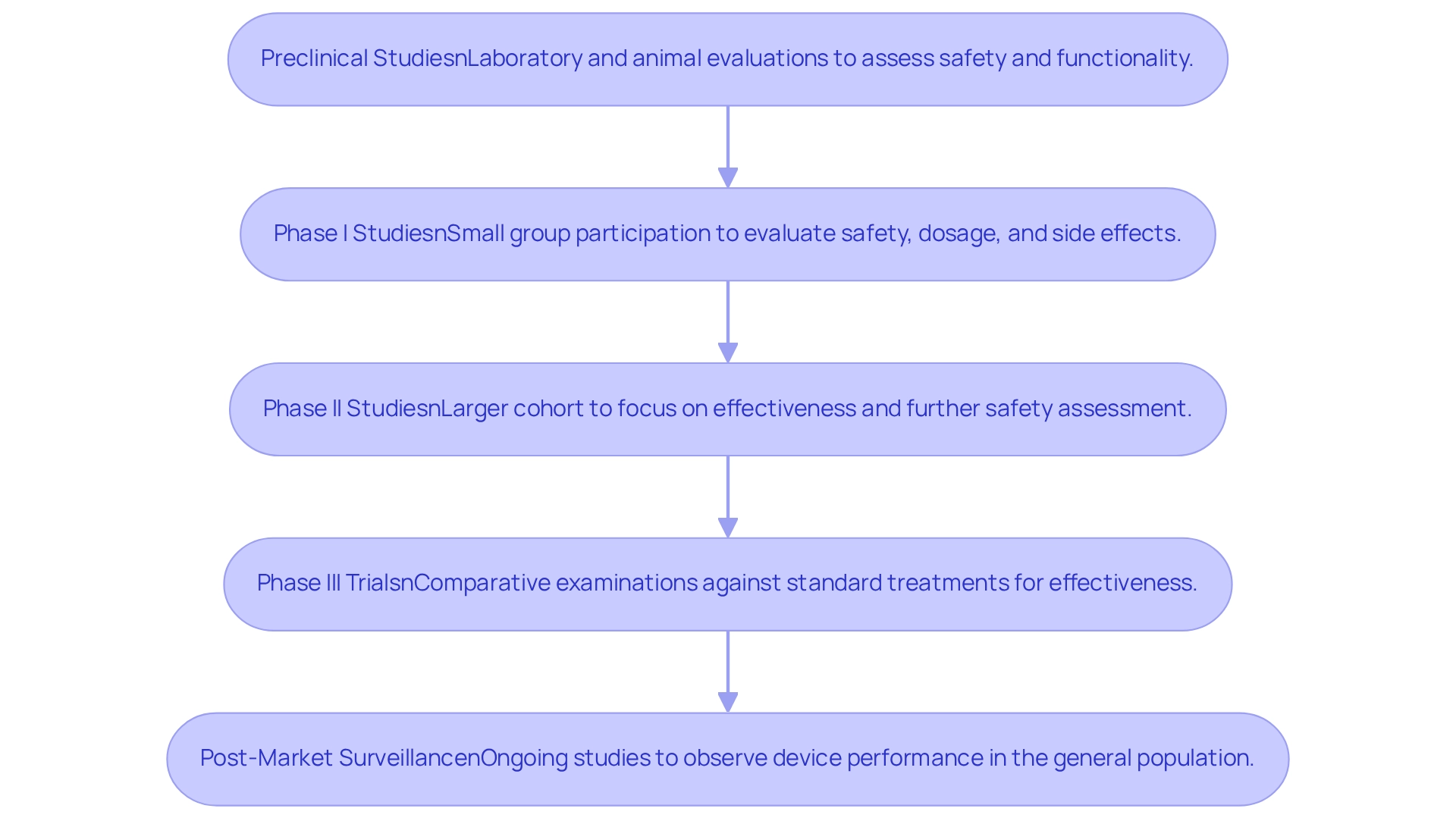
Designing clinical trials for medical devices in Bolivia involves systematic investigations that evaluate the safety and effectiveness of innovative technologies. These studies typically progress through several critical phases.
- Preclinical Studies involve laboratory and animal evaluations to assess safety and functionality before human testing.
- Phase I Studies engage a small group of participants to evaluate safety, determine dosage, and identify potential side effects.
- Phase II Studies are conducted with a larger cohort, focusing on the device's effectiveness while further assessing its safety.
- Phase III Trials serve as crucial examinations, comparing the new device against standard treatments to verify effectiveness and monitor adverse reactions.
- Finally, Post-Market Surveillance involves ongoing studies that observe the device's performance in the general population, ensuring continued safety and effectiveness.
At bioaccess®, we specialize in comprehensive research management services tailored for the Latin American market. Our expertise encompasses Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Understanding these stages is essential for designing clinical trials for medical devices in Bolivia, ensuring they comply with regulatory standards and achieve significant outcomes. By leveraging our extensive knowledge and customized strategies, we assist in navigating the complexities of the Latin American Medtech landscape, ensuring positive results for your research initiatives.
Identify Regulatory Requirements for Clinical Trials in Bolivia
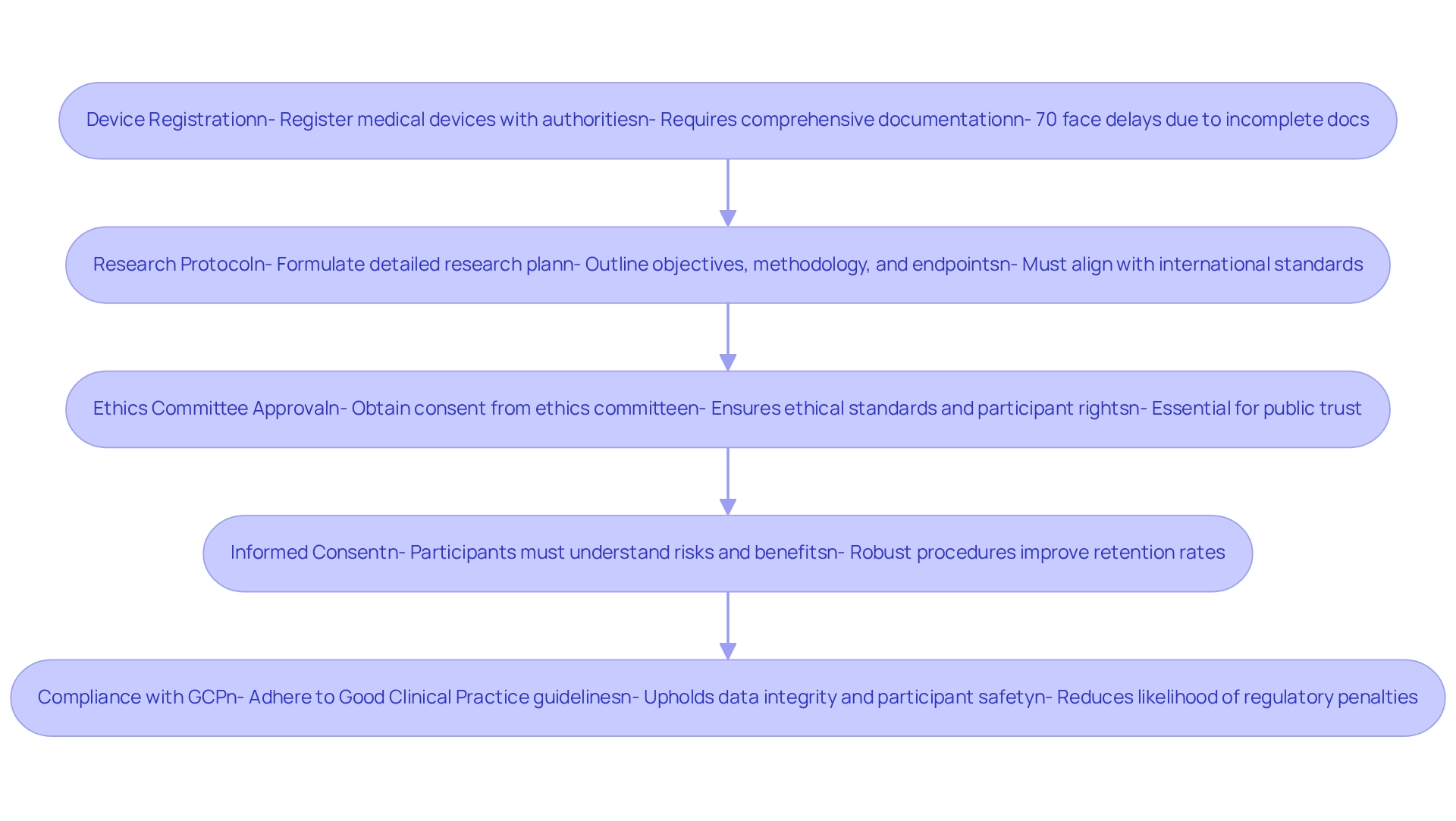
Designing clinical trials for medical devices in Bolivia requires strict compliance with the regulatory framework established by the Ministry of Health and the National Pharmacology and Health Technologies Agency. The following key steps delineate the process:
-
Device Registration: Prior to initiating the study, medical devices must be registered with the appropriate authorities. This registration demands comprehensive documentation that substantiates the device's safety and efficacy. Recent statistics indicate that approximately 70% of medical devices submitted for registration in Bolivia encounter delays due to incomplete documentation.
-
Research Protocol: A detailed research protocol must be formulated, clearly outlining the project's objectives, methodology, and endpoints. This plan is subject to regulatory approval. Experts recommend that this plan aligns with international standards to facilitate smoother approval processes.
-
Ethics Committee Approval: Securing consent from an ethics committee is vital to ensure that the study adheres to ethical standards and protects participant rights. Experts underline that this step is essential for maintaining public trust and ensuring participant welfare. As Dr. Maria Lopez, a regulatory specialist, asserts, "Ethics committee approval is not merely a formality; it is a cornerstone of ethical clinical research that safeguards participants and boosts the credibility of the project."
-
Informed Consent: It is imperative that all participants provide informed consent, fully understanding the associated risks and benefits of their involvement in the study. Recent case studies suggest that studies with robust informed consent procedures report higher participant retention rates.
-
Compliance with Good Clinical Practice (GCP): Throughout the study, adherence to GCP guidelines is mandatory to uphold data integrity and ensure participant safety. A recent analysis emphasized that compliance with GCP significantly reduces the likelihood of regulatory penalties.
In addition to these measures, leveraging the expertise of bioaccess® can significantly enhance the research process. With over 20 years of experience in Medtech, bioaccess® specializes in managing various research projects, including Early-Feasibility Assessments (EFS), First-In-Human Trials (FIH), Pilot Evaluations, Pivotal Trials, and Post-Market Clinical Follow-Up Evaluations (PMCF). Their extensive management services encompass feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting on study progress and adverse events, ensuring a streamlined approach to navigating the complexities of research in Bolivia.
Understanding these regulatory requirements is crucial for effectively navigating the research landscape in Bolivia, particularly in the context of designing clinical trials for medical devices in Bolivia, as regulations evolve in 2025 to enhance their safety and efficacy. Reach out to learn how we can assist with your medical studies.
Design the Clinical Trial Protocol and Methodology
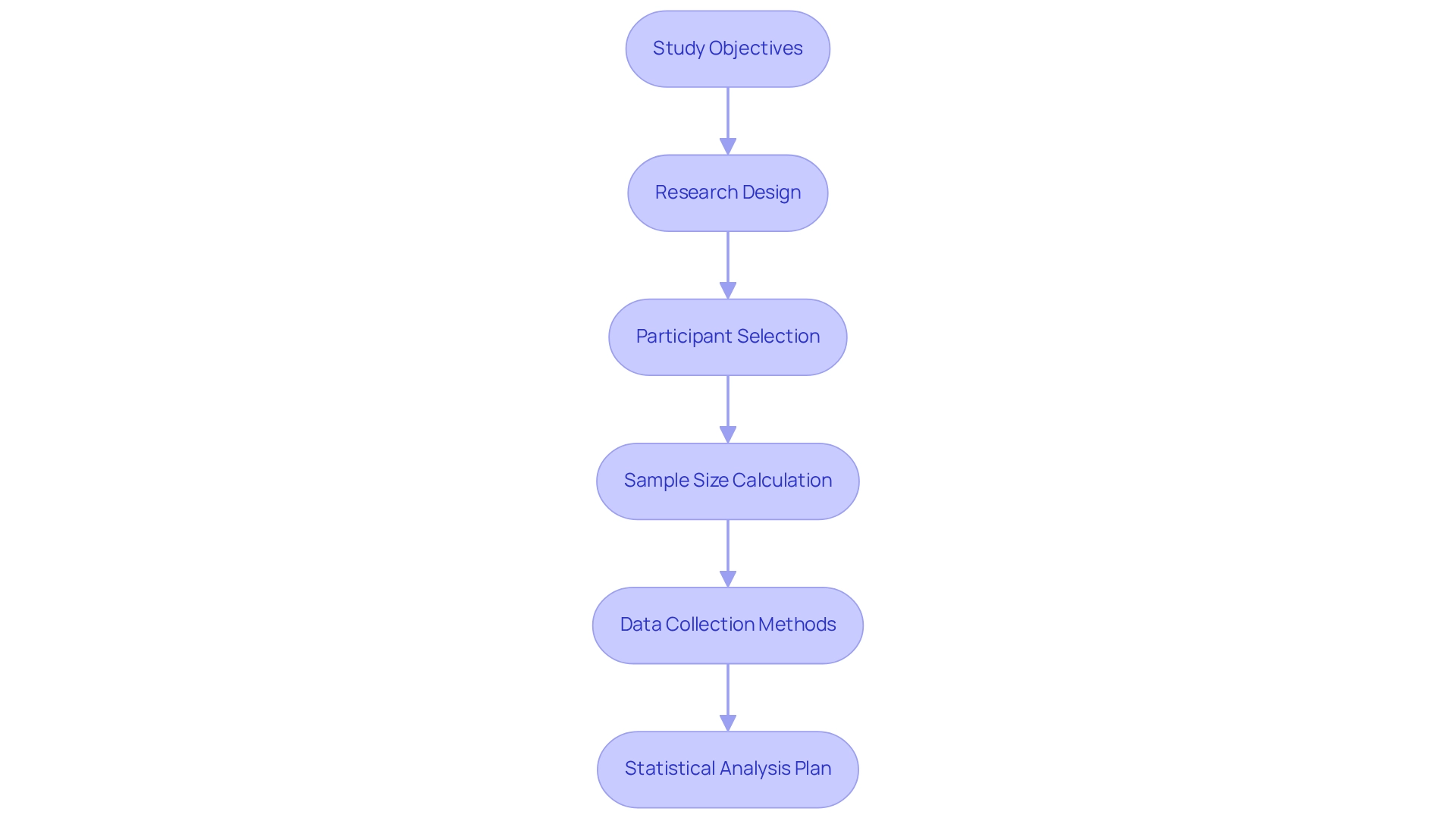
Designing a clinical trial protocol for medical devices involves several critical components that ensure the study's success and regulatory compliance:
-
Study Objectives: Clearly define the primary and secondary goals of the study. Determining what questions the research intends to address is essential for directing the whole research process.
-
Research Design: Choose a suitable design, like a randomized controlled trial or observational analysis, customized to the goals and the particular features of the device being evaluated. This choice significantly impacts the reliability of the results.
-
Participant Selection: Define inclusion and exclusion criteria meticulously to identify suitable participants. This step is essential for ensuring that the research population is representative, which improves the generalizability of the findings. The FDA stresses that clinical trials involving varied participants produce findings relevant to the wider patient population, underscoring the significance of inclusivity in participant selection. Moreover, the FDA demands inclusion of various age categories, particularly in pediatric research, because of varying health requirements and treatment reactions.
-
Sample Size Calculation: Accurately determine the number of participants required to achieve statistically significant results. This calculation should consider the anticipated effect size and variability, ensuring that the research is adequately powered to detect meaningful differences.
-
Data Collection Methods: Specify the methods for data collection, detailing the types of measurements and assessments that will be utilized. This clarity is essential for maintaining consistency and reliability throughout the research.
-
Statistical Analysis Plan: Outline the statistical methods that will be employed to analyze the data, ensuring alignment with the study objectives. A well-defined analysis plan is vital for interpreting results accurately and drawing valid conclusions.
Incorporating bioaccess's comprehensive clinical study management services can significantly improve the design and execution of these protocols. With over 20 years of experience in Medtech, bioaccess specializes in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies. Their adaptability and expert knowledge guarantee that every element of the evaluation, from feasibility assessments to compliance reviews and project management, is managed with accuracy.
A comprehensive protocol is essential when designing clinical trials for medical devices in Bolivia, as it not only guides the research team but also serves as a critical document for regulatory submissions. Incorporating best practices in research methodology, such as community engagement strategies, can further enhance participant recruitment and retention, particularly among underrepresented populations. For example, effective approaches for community involvement can boost trust and participation in research, resulting in more representative and dependable data. By concentrating on these factors, researchers can create impactful studies that provide valuable insights to the Medtech sector. As the FDA states, "Clinical studies that include diverse participants are more likely to yield results that are applicable to the entire patient population." This highlights the significance of diversity in health studies, particularly considering wider health equity issues.
Develop Effective Recruitment Strategies for Participants
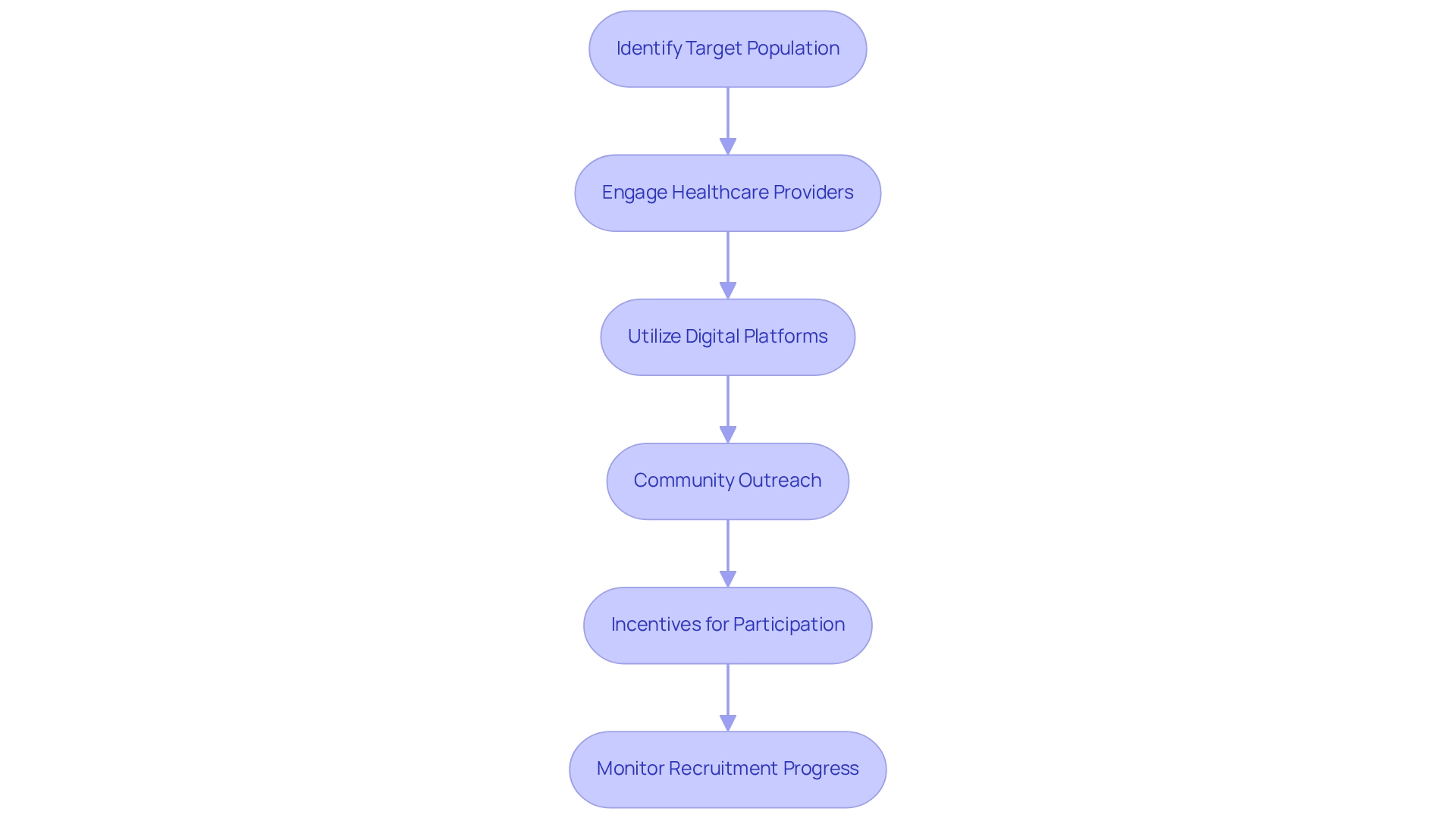
Developing effective recruitment strategies for clinical trials necessitates a nuanced understanding of the target population and the implementation of diverse outreach methods.
- Identify Target Population: Gain insights into the demographics and characteristics of individuals who would benefit from the medical device. Tailor recruitment efforts to resonate with these specific groups, particularly considering that 18 percent of the U.S. population identifies as Hispanic/Latino(a)—a demographic that may reflect similar trends in Latin America.
- Engage Healthcare Providers: Collaborate with local healthcare providers, who can serve as reliable sources of information, to inform potential participants about the study. This approach not only builds credibility but also enhances recruitment effectiveness. As one Study Coordinator noted, "It is a very slow process. So we started with one clinic that we had done a lot of formative work and we partnered with them for some time." This strategy is especially pertinent in areas such as Barranquilla, where bioaccess™ and Caribbean Health Group are collaborating to establish the city as a prominent location for medical research in Latin America, with the backing of Colombia's Minister of Health.
- Utilize Digital Platforms: Harness the power of social media and online platforms to broaden outreach. Develop captivating material that clearly expresses the objective and possible advantages, ensuring it is reachable for a broader audience.
- Community Outreach: Partner with community organizations and patient advocacy groups to raise awareness and foster participation. Such partnerships can greatly improve trust and interest in the study, reflecting the endeavors of bioaccess™ to involve local communities in research initiatives.
- Incentives for Participation: Consider offering incentives, such as travel reimbursement or complimentary health screenings, to motivate participation. This strategy can be particularly effective in increasing enrollment rates.
- Monitor Recruitment Progress: Continuously assess recruitment efforts and be ready to adapt strategies if enrollment targets are not being met. This proactive approach ensures that recruitment remains on track.
By employing these strategies, researchers can significantly enhance participant recruitment, ultimately aiding in the success of their research studies. Notably, studies indicate that a substantial percentage of participants are recruited through healthcare providers, underscoring the importance of these partnerships. Furthermore, innovative methodologies, such as the systematic randomized qualitative assessment methodology, have shown promise in improving recruitment strategies, particularly for underrepresented groups. Efficient involvement of healthcare professionals can lead to more inclusive and representative research studies, which is crucial for advancing medical technology. Moreover, comprehending the external motivations for diversity in studies, such as funding agency requirements, can further inform recruitment strategies. The collaboration between bioaccess™ and Caribbean Health Group exemplifies how strategic partnerships can enhance clinical trial outcomes and contribute to local economic growth, highlighting the comprehensive clinical trial management services offered by bioaccess™.
Conclusion
The intricate journey of conducting clinical trials for medical devices is a multifaceted process that demands careful attention to detail at every stage. From foundational preclinical studies to post-market surveillance, each phase plays a pivotal role in ensuring that new technologies are both safe and effective. Understanding the regulatory landscape in Bolivia is crucial, as compliance with local laws can significantly influence trial outcomes. By adhering to established protocols and regulations, researchers can navigate the complexities of clinical trials more successfully.
Designing a robust clinical trial protocol is essential for achieving meaningful results. This involves clearly defining study objectives, selecting appropriate methodologies, and ensuring ethical standards are met. Moreover, effective recruitment strategies are vital for engaging participants, particularly in diverse populations. Collaborating with healthcare providers, leveraging digital platforms, and fostering community outreach can enhance recruitment efforts, ultimately leading to more representative and impactful clinical studies.
As the medical technology landscape continues to evolve, so too must the strategies employed in clinical trials. By focusing on comprehensive planning, regulatory compliance, and innovative recruitment techniques, researchers can significantly improve the success rates of their trials. The insights provided in this article serve as a valuable guide for navigating the complexities of clinical trials in Bolivia and beyond, emphasizing the importance of meticulous preparation and strategic engagement in advancing medical innovation.
Frequently Asked Questions
What are the main phases of clinical trials for medical devices in Bolivia?
The main phases of clinical trials for medical devices in Bolivia include Preclinical Studies, Phase I Studies, Phase II Studies, Phase III Trials, and Post-Market Surveillance.
What is involved in Preclinical Studies?
Preclinical Studies involve laboratory and animal evaluations to assess the safety and functionality of the device before it undergoes human testing.
What is the purpose of Phase I Studies?
Phase I Studies engage a small group of participants to evaluate the safety of the device, determine appropriate dosage, and identify potential side effects.
How do Phase II Studies differ from Phase I Studies?
Phase II Studies are conducted with a larger group of participants, focusing on the effectiveness of the device while further assessing its safety.
What occurs during Phase III Trials?
Phase III Trials serve as crucial examinations that compare the new device against standard treatments to verify its effectiveness and monitor any adverse reactions.
What is the role of Post-Market Surveillance?
Post-Market Surveillance involves ongoing studies that observe the device's performance in the general population, ensuring its continued safety and effectiveness.
What services does bioaccess® provide for clinical trials in Latin America?
Bioaccess® specializes in comprehensive research management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
Why is it important to understand the stages of clinical trials for medical devices?
Understanding these stages is essential for designing clinical trials that comply with regulatory standards and achieve significant outcomes in Bolivia's Medtech landscape.




