Overview
This article examines the critical steps in designing clinical trials for medical devices in Ecuador, underscoring the necessity of grasping the regulatory landscape and executing effective strategies throughout the trial process. It is essential to adhere to the updated regulations from ARCSA, engage in thorough planning, and collaborate with seasoned organizations such as bioaccess® to guarantee the success and compliance of these clinical studies.
Introduction
In the dynamic landscape of medical device development, understanding the regulatory framework in Ecuador is paramount for designing effective clinical trials. Recent updates introduced by the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA) necessitate that stakeholders navigate a complex set of guidelines dictating every aspect of trial approval and execution.
From defining research questions to ensuring ethical compliance, each step in the trial process is critical for success. As the demand for innovative medical solutions grows, particularly in the context of Ecuador's expanding healthcare sector, the need for comprehensive strategies becomes increasingly evident.
This article delves into the essential steps for designing clinical trials, effective recruitment strategies, and the importance of monitoring compliance, while highlighting the role of experienced partners like bioaccess® in facilitating these processes.
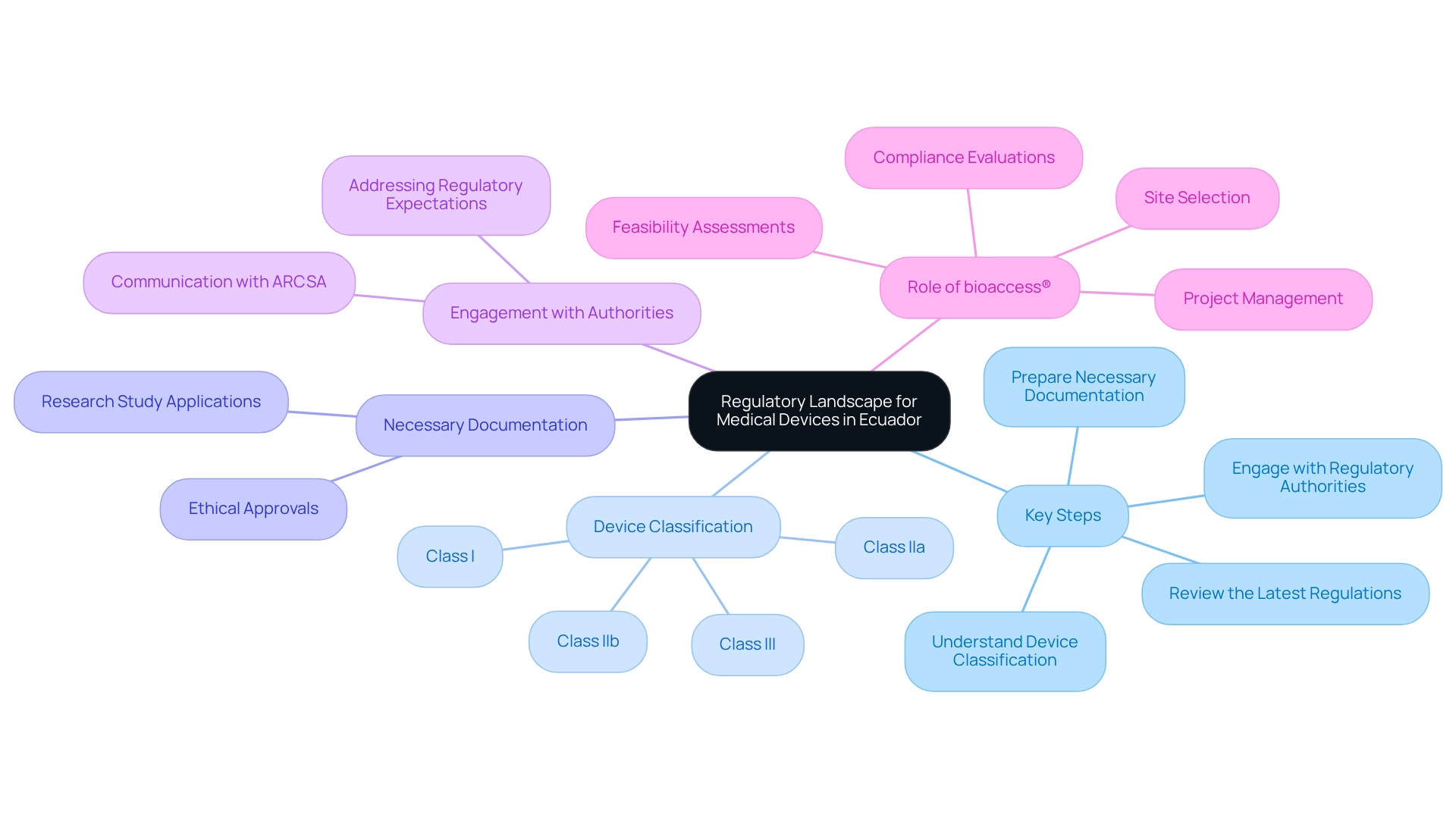
Understand the Regulatory Landscape for Medical Devices in Ecuador
Understanding the regulatory framework established by the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA) is crucial for designing clinical trials for medical devices in Ecuador. The recent updates to regulations, particularly those implemented in March 2025, provide comprehensive guidelines for designing clinical trials for medical devices in Ecuador, covering approval, authorization, execution, oversight, and management of research studies. Key steps to consider include:
- Review the Latest Regulations: Regularly consult the most recent regulations and guidelines issued by ARCSA, including any amendments that may influence research protocols.
- Understand Device Classification: Medical devices in Ecuador are classified according to risk levels (Class I, IIa, IIb, and III), each with specific compliance requirements that must be adhered to.
- Prepare Necessary Documentation: Ensure that all required documentation, such as research study applications and ethical approvals, is meticulously prepared in accordance with ARCSA guidelines.
- Engage with Regulatory Authorities: Initiate communication with ARCSA early in the process to address any uncertainties regarding regulatory expectations and requirements.
In this context, collaborating with bioaccess® can significantly enhance the research process. With over 20 years of experience in Medtech, bioaccess® offers extensive management services for research projects, including feasibility assessments, site selection, compliance evaluations, project setup, import permits, project management, and reporting. Their expertise encompasses various study types, such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). The recent economic expansion in Ecuador has led to increased healthcare spending, creating a favorable environment for research studies. This economic context underscores the importance of adhering to the new regulations, which aim to enhance the rigor, transparency, and impact of clinical studies, ultimately benefiting the health and welfare of the population, particularly in the context of designing clinical trials for medical devices in Ecuador. As Dr. Patricia Saidón from the Ministry of Health emphasized, the new regulation is vital for conducting rigorous, transparent, and impactful studies on the health and welfare of populations. Furthermore, training programs focused on ethical and regulatory aspects have been initiated to support the successful implementation of these regulations, ensuring stakeholders are well-equipped to navigate this evolving landscape.
Outline Key Steps in Designing Clinical Trials for Medical Devices
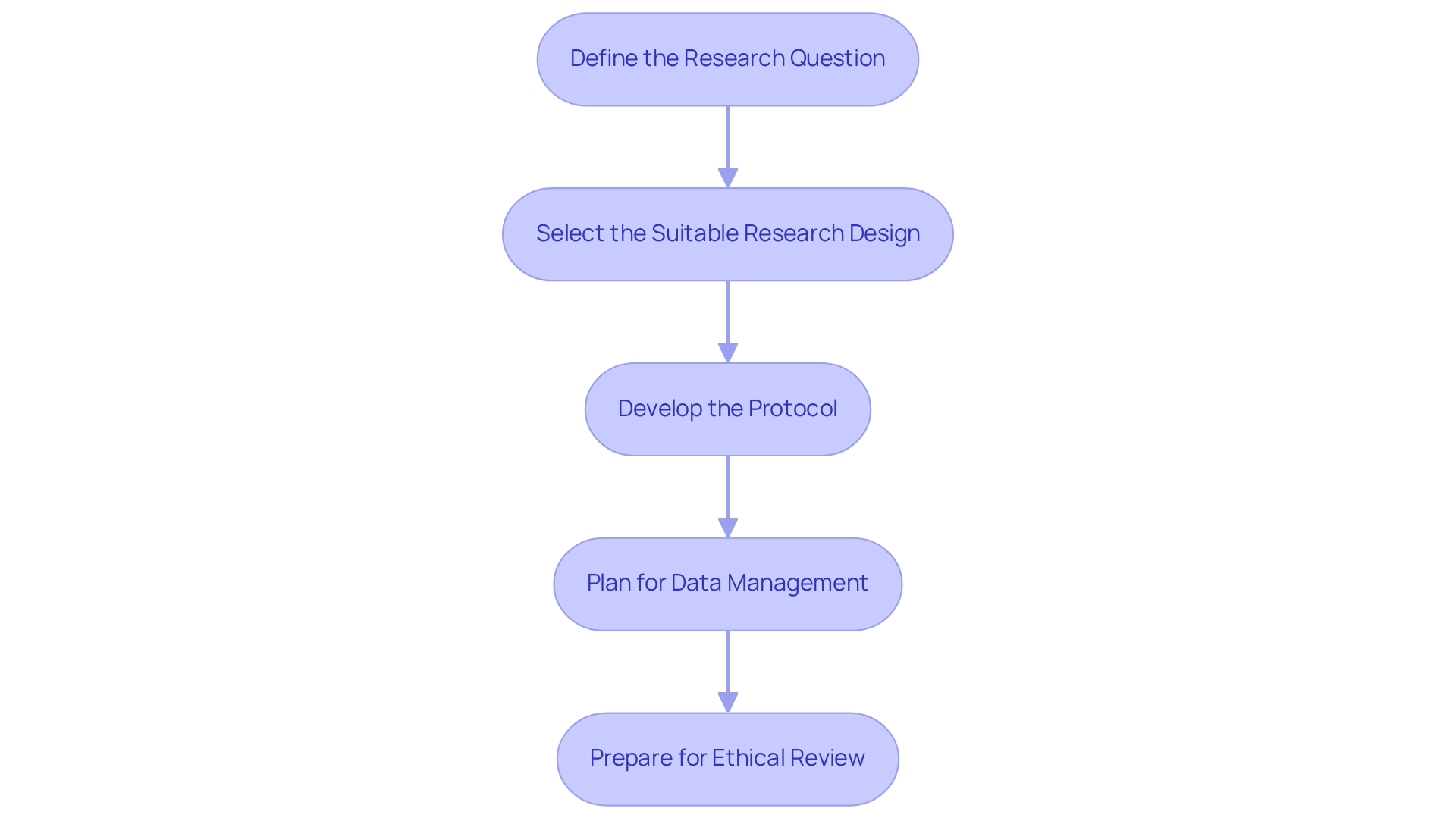
Designing clinical trials for medical devices in Ecuador necessitates a systematic approach that includes several key steps.
- Define the Research Question: Clearly articulate the trial's objectives, specifying both primary and secondary endpoints. This foundational step guarantees that the research remains focused and pertinent to the intended outcomes.
- Select the Suitable Research Design: Choose a research design that best aligns with the research question. Common choices consist of randomized controlled experiments, cohort studies, and case-control studies. Each design has its strengths and should be selected based on the specific context of the trial.
- Develop the Protocol: Create a comprehensive research protocol detailing the methodology, participant eligibility criteria, intervention specifics, and statistical analysis plan. A well-organized protocol is essential for directing the study and ensuring adherence to legal standards.
- Plan for Data Management: Establish a robust data management plan that outlines data collection methods, storage solutions, and analysis procedures. This step is vital for maintaining data integrity and ensuring adherence to regulatory requirements.
- Prepare for Ethical Review: Submit the study protocol to an ethics committee for thorough review and approval. Addressing all ethical considerations is essential to uphold the integrity of the research and protect participant welfare.
The FDA highlights that adaptable design and analysis can guarantee more patients obtain effective therapies ethically without compromising the integrity of research.
Including best practices, such as acknowledging the variations in baseline covariates that can influence the exchangeability of trials, improves the design process. For instance, after trimming, 290 current patients and 941 external patients were included and divided into five PS strata, highlighting the importance of robust patient selection.
Moreover, the increasing interest in Bayesian techniques, which provide adaptability and clear interpretations, is transforming study designs, especially for innovative medical products. A case study comparing Bayesian and Frequentist approaches illustrates this trend, noting that while the latter has been more commonly used due to its established statistical properties, the Bayesian method offers more intuitive interpretations of research outcomes.
By adhering to these steps and utilizing expert knowledge from bioaccess®, which focuses on Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, and boasts over 20 years of experience in Medtech, researchers can effectively navigate the intricacies of designing clinical trials for medical devices in Ecuador.
Implement Effective Recruitment and Site Selection Strategies
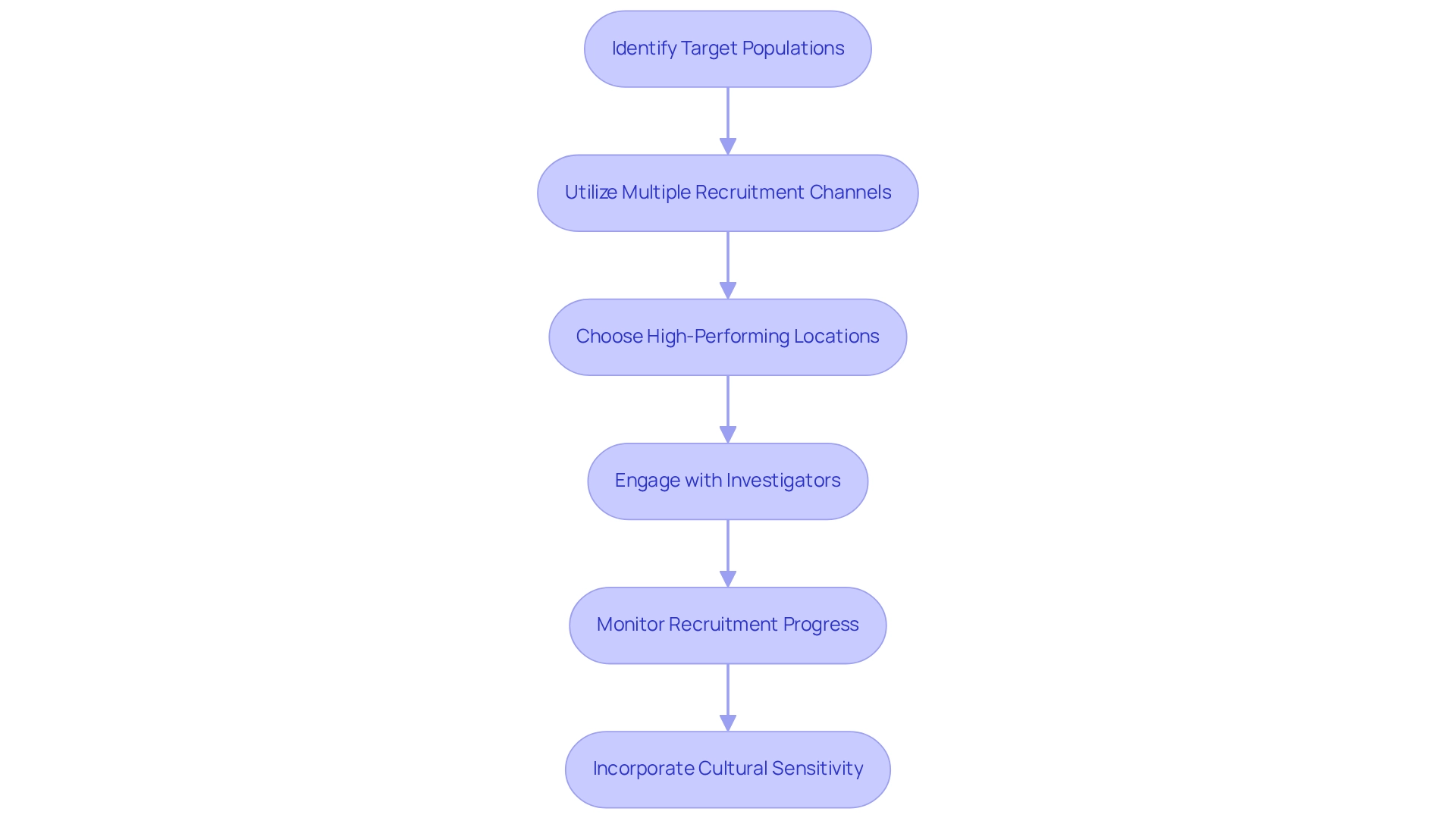
To ensure the success of your clinical trial, designing clinical trials for medical devices in Ecuador involves implementing effective recruitment and site selection strategies tailored to the unique landscape of Latin America.
- Identify Target Populations: Clearly defining the characteristics of ideal participants based on the study's objectives and specific inclusion/exclusion criteria is crucial. Understanding local demographics and health profiles will significantly enhance recruitment efforts in designing clinical trials for medical devices in Ecuador.
- Utilize Multiple Recruitment Channels: Employing a diverse array of recruitment methods—such as social media campaigns, community outreach initiatives, and collaborations with healthcare providers—enhances visibility and engagement with potential participants. This multifaceted approach is vital for designing clinical trials for medical devices in Ecuador to effectively reach diverse populations.
- Choose High-Performing Locations: Prioritizing research sites with a proven history of conducting successful studies is key. Factors such as infrastructure, staff expertise, and previous enrollment success rates should guide your selections. For instance, bioaccess™ has collaborated with Caribbean Health Group to establish Barranquilla as a prominent location for clinical studies in Latin America, supported by Colombia's Minister of Health.
- Engage with Investigators: Cultivating strong relationships with principal investigators and site staff is essential. Their dedication and teamwork are crucial for sustaining progress throughout the experiment and ensuring compliance with protocols. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human testing in Colombia, underscoring the significance of robust investigator engagement.
- Monitor Recruitment Progress: Continuously evaluating recruitment metrics and being prepared to adjust strategies as necessary is vital. This proactive approach aids in achieving enrollment goals and can significantly improve the overall effectiveness of the study. GlobalCare Clinical Studies has partnered with bioaccess™ to enhance ambulatory services for research in Colombia, achieving over a 50% decrease in recruitment time and 95% retention rates.
Incorporating cultural sensitivity into the recruitment process is essential when designing clinical trials for medical devices in Ecuador. For example, understanding that patients in Latin America may accept physician recommendations without question can complicate informed consent. The case analysis titled "Cultural Sensitivity in Informed Consent" highlights how these cultural differences affect patient perceptions and emphasizes the need to reconcile local practices while respecting patient rights. Therefore, designing clinical trials for medical devices in Ecuador must prioritize ensuring ethical compliance.
Success narratives in enrollment can inspire future involvement and enhance morale among research teams, emphasizing the significance of efficient recruitment strategies in reaching study objectives. Companies like bioaccess® utilize feasibility assessments and cost-efficient hiring strategies tailored to local dynamics, engaging communities to improve awareness and trust. With over 15 years of experience in the Medtech sector, bioaccess® exemplifies the expertise necessary to navigate these challenges effectively.
Monitor and Adapt Trial Protocols for Compliance and Efficiency
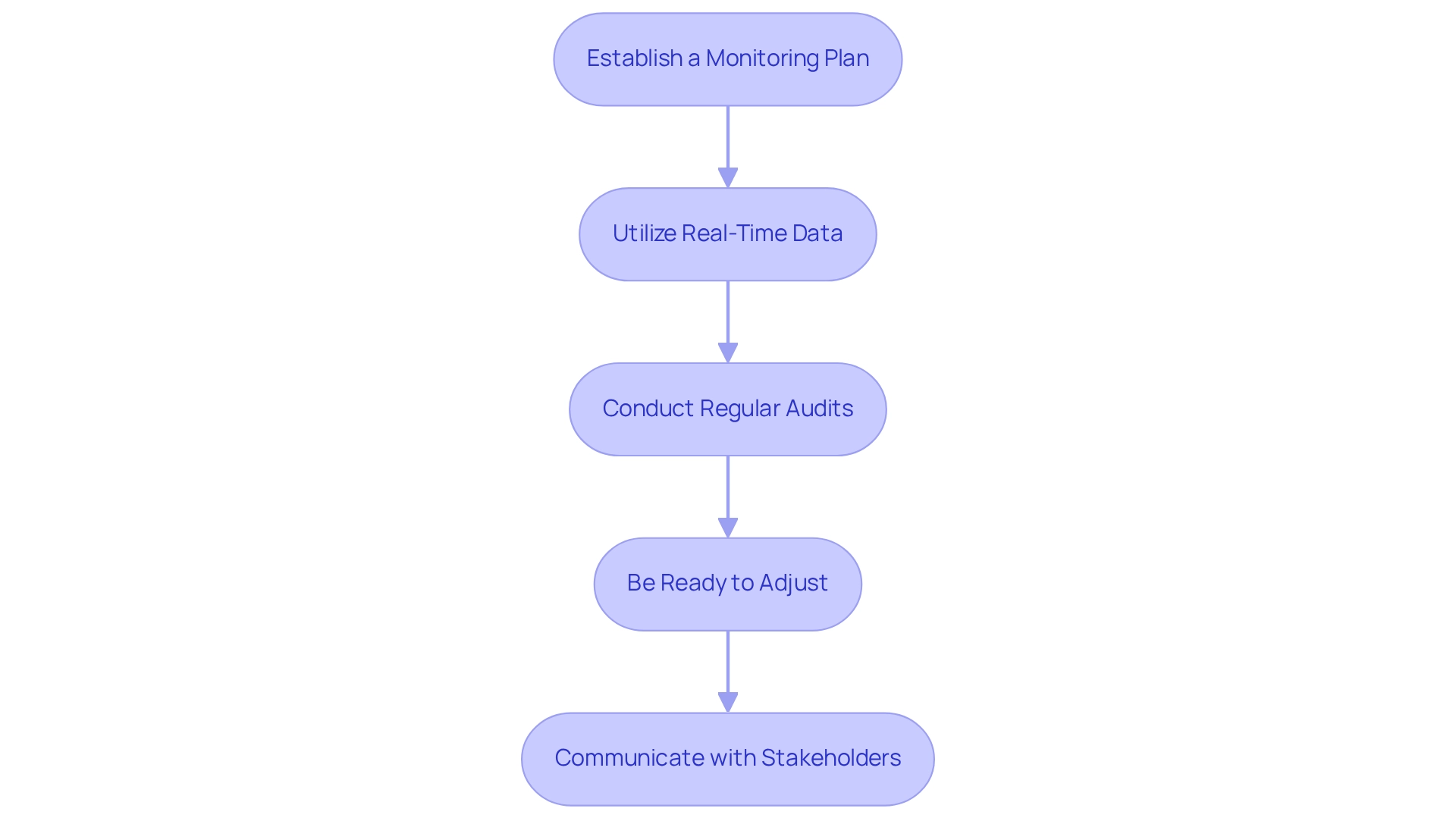
To ensure adherence and efficiency in designing clinical trials for medical devices in Ecuador, implementing robust monitoring and adaptation strategies is essential.
-
Establish a Monitoring Plan: A detailed monitoring plan should be created, specifying the frequency and methods for monitoring activities, including site visits and data reviews. This plan must be customized to the distinct features of the examination and the regulatory environment in Latin America, particularly considering the oversight provided by INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a crucial role in medical device regulation.
-
Utilize Real-Time Data: Advanced technologies should be harnessed to collect and analyze data in real-time. This method allows for the prompt detection of problems and patterns, enabling proactive modifications to the experiment as necessary. In 2025, the integration of real-time data collection is becoming increasingly vital for enhancing study efficiency and compliance, especially in the context of accelerated medical device clinical study services offered by bioaccess®.
-
Conduct Regular Audits: A timetable for routine evaluations of procedures and data must be established. These audits are vital for ensuring adherence to established protocols and legal requirements, thereby minimizing risks associated with non-compliance. Bioaccess® highlights the significance of thorough clinical study management services, including compliance reviews, to maintain the highest standards.
-
Be Ready to Adjust: Protocols should remain adaptable to accommodate interim findings, participant feedback, or changes in compliance guidelines. Adaptive research designs can significantly improve results by enabling researchers to react efficiently to emerging data, a strategy backed by bioaccess®'s expertise in managing various research types, including Early-Feasibility and Pivotal Investigations.
-
Communicate with Stakeholders: Open communication channels with all stakeholders, including regulatory bodies and study participants, must be fostered. Quickly addressing compliance concerns through open dialogue can reduce potential issues and improve trust in the process. The partnership between bioaccess™ and local health institutions, such as the Caribbean Health Group, illustrates the significance of stakeholder involvement in establishing Barranquilla as a premier destination for medical studies in Latin America.
Evidence shows that numerous compliance-related issues from on-site monitoring can also be identified through centralized statistical monitoring (CSM) techniques, emphasizing the necessity of combining various monitoring approaches. Furthermore, an examination of 158 randomized controlled studies concerning device interventions revealed that merely 9% of investigations incorporated adherence as a variable in the analysis, highlighting the necessity for increased awareness and utilization of statistical techniques to account for adherence in research. By adopting these strategies, clinical trials can achieve higher levels of compliance and efficiency, ultimately leading to more successful outcomes.
Conclusion
Navigating the regulatory landscape for medical device development in Ecuador is essential for the success of clinical trials. Recent updates from ARCSA have established a comprehensive framework that stakeholders must adhere to, which includes a thorough understanding of device classifications and the preparation of necessary documentation. Collaborating with experienced partners like bioaccess® can significantly streamline compliance with these guidelines.
A systematic approach to trial design is imperative, encompassing clear research questions, appropriate study designs, and thorough protocols. Incorporating best practices in data management and ethical review is crucial for maintaining research integrity, while innovative methodologies, such as Bayesian approaches, enhance flexibility in interpreting outcomes.
Effective recruitment and site selection play a critical role in the success of trials. Identifying target populations, utilizing diverse recruitment strategies, and engaging skilled investigators can greatly improve participant retention and overall efficiency. Continuous monitoring of recruitment progress ensures that enrollment targets are consistently met.
Implementing a detailed monitoring plan and leveraging real-time data enhances compliance and efficiency throughout the trial process. Open communication with stakeholders and adaptability to changes are key to addressing compliance concerns.
In conclusion, conducting successful clinical trials in Ecuador necessitates a thorough understanding of the regulatory framework, strategic planning, effective recruitment, and robust monitoring practices. By prioritizing these elements and partnering with experienced organizations like bioaccess®, stakeholders can enhance trial outcomes and contribute to advancements in healthcare solutions that benefit the population.
Frequently Asked Questions
Why is understanding the regulatory framework established by ARCSA important for clinical trials in Ecuador?
Understanding the regulatory framework established by the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA) is crucial because it provides comprehensive guidelines for the approval, authorization, execution, oversight, and management of clinical trials for medical devices in Ecuador.
What are the key steps to consider when designing clinical trials for medical devices in Ecuador?
Key steps include reviewing the latest regulations from ARCSA, understanding device classification according to risk levels, preparing necessary documentation in accordance with ARCSA guidelines, and engaging with regulatory authorities early in the process.
How are medical devices classified in Ecuador?
Medical devices in Ecuador are classified according to risk levels into four classes: Class I, IIa, IIb, and III, each with specific compliance requirements.
What kind of documentation is required for clinical trials?
Required documentation includes research study applications and ethical approvals, which must be meticulously prepared according to ARCSA guidelines.
How can engaging with ARCSA benefit the research process?
Initiating communication with ARCSA early helps address uncertainties regarding regulatory expectations and requirements, facilitating a smoother research process.
What services does bioaccess® provide for research projects?
Bioaccess® offers extensive management services for research projects, including feasibility assessments, site selection, compliance evaluations, project setup, import permits, project management, and reporting.
What types of studies does bioaccess® support?
Bioaccess® supports various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
How has the recent economic expansion in Ecuador affected healthcare spending?
The recent economic expansion has led to increased healthcare spending, creating a favorable environment for conducting research studies.
Why are the new regulations from ARCSA important?
The new regulations aim to enhance the rigor, transparency, and impact of clinical studies, ultimately benefiting the health and welfare of the population.
What initiatives are in place to support the implementation of the new regulations?
Training programs focused on ethical and regulatory aspects have been initiated to ensure stakeholders are well-equipped to navigate the evolving regulatory landscape.




