Overview
Designing clinical trials for medical devices in Paraguay is a critical endeavor that necessitates a thorough understanding of the regulatory landscape. This process involves several key steps:
- Defining objectives
- Selecting appropriate research designs
- Obtaining the necessary ethical and regulatory approvals
Compliance with DINAVISA regulations and international standards cannot be overstated, as it forms the backbone of successful trial execution. Furthermore, effective recruitment strategies are essential to enhance participant enrollment, which ultimately promotes successful trial outcomes and advances medical technology in the region. As we navigate these complexities, collaboration among stakeholders becomes increasingly vital, ensuring that we address the unique challenges faced in clinical research.
Introduction
Navigating the complex world of clinical trials, particularly for medical devices, necessitates a profound understanding of regulatory frameworks, ethical considerations, and effective recruitment strategies. In Paraguay, the landscape is significantly influenced by the guidelines established by DINAVISA, which dictate approval processes and ensure participant safety.
As interest in clinical research surges—especially in response to recent global health challenges—researchers must adeptly adapt to evolving regulations and innovative methodologies. This article explores the essential steps for designing successful clinical trials, including:
- Comprehending the regulatory environment
- Implementing post-market follow-up studies
These steps are all aimed at enhancing the efficacy and safety of medical devices in Latin America. With insights into effective participant recruitment and compliance strategies, it provides a comprehensive roadmap for researchers seeking to excel in this dynamic field.
Understand the Regulatory Landscape for Clinical Trials in Paraguay
Designing clinical trials for medical devices in Paraguay requires a comprehensive understanding of the regulatory framework established by DINAVISA (National Directorate of Vigilance Sanitaria). Familiarity with the research approval process is essential, as it typically requires the submission of extensive documentation outlining the study's objectives, methodologies, and ethical considerations. Key regulations to consider include:
- Resolution 614: This regulation delineates the procedures for obtaining research study approval, detailing the necessary documentation and anticipated timelines for review. Ethical Guidelines: Adherence to ethical standards is crucial, particularly regarding informed consent and participant safety throughout the trial.
- Global Standards: Compliance with international guidelines, such as Good Clinical Practice (GCP), is vital, as these may influence local regulatory requirements.
Recent trends indicate a growing interest in research involving human participants within Paraguay, evident in the significant increase in submissions during 2018 and 2019. A DINAVISA associate noted that the number of research submissions surged during this period. However, the total number of analyzed submissions by Ethics Review Boards (ERBs) remained below 50 research projects from 2015 to 2020. This underscores the need for a cultural transformation in science policy formulation to prioritize long-term research objectives, which is essential for enhancing regulatory approval processes.
The COVID-19 pandemic has further stimulated growth in medical research opportunities, particularly in studies related to COVID-19 therapies and vaccines. This surge has led to the establishment of recognized ERBs and improved collaboration among healthcare professionals and researchers, facilitating the approval of more successful studies in the region. By efficiently navigating these regulatory obligations, researchers can mitigate potential setbacks and streamline the approval process for designing clinical trials for medical devices in Paraguay. Partnering with bioaccess® can significantly enhance this process, as their expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot, Pivotal, and Post-Market Follow-Up Studies ensures that all regulatory and compliance aspects are meticulously handled, ultimately resulting in improved timelines and success rates in approvals.
Outline the Key Steps in Designing Clinical Trials for Medical Devices
Designing clinical trials for medical devices in Paraguay requires a methodical strategy to ensure effectiveness and compliance. With over 200,000 medical device professionals engaged in this field, understanding the key steps is crucial. Here are the essential steps to consider:
- Define Objectives: Clearly articulate the primary and secondary goals of the experiment. Comprehending what you intend to demonstrate or assess is essential for directing the research's path.
- Select Research Design: Choose a suitable research framework, such as a randomized controlled experiment or observational analysis, tailored to your objectives and the specific characteristics of the device.
- Determine Sample Size: Calculate the necessary sample size to achieve statistical significance. This calculation should factor in the expected effect size, variability, and additional considerations such as test margin, subject drop-out rate, and treatment allocation. As Jon Bergsteinsson mentions, "When it comes to conducting studies aimed at validating a theory or assertion for therapeutic performance or safety, such as pivotal studies, research for market access, or PMCF, you will need to provide justification for sample size calculation."
- Develop Protocol: Create a comprehensive protocol detailing the methodology, participant criteria, data collection methods, and analysis plans. A well-organized protocol is crucial for directing the study and ensuring consistency.
- Ethics Approval: Submit the protocol to an ethics committee for approval. Responsible conduct of clinical studies necessitates adherence to ethical standards, prioritizing the rights and welfare of participants. This involves obtaining informed consent and ensuring continuous monitoring throughout the study, as highlighted in the case study 'Ensuring Ethical Considerations.'
- Regulatory Submission: Prepare and submit the necessary documentation to DINAVISA for regulatory approval. This step is critical for ensuring compliance with local regulations and facilitating the trial's progression. Understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is essential, as it oversees medical device regulation and classification as a Level 4 health authority by PAHO/WHO.
- Maintain Best Practices: Throughout the study, it is essential to maintain best practices and critical thinking when applying risk assessment principles. This method guarantees that the study design remains robust and ethically sound.
By adhering to these steps, researchers can ensure that designing clinical trials for medical devices in Paraguay leads to medical studies that are not only methodologically sound but also ethically responsible, ultimately promoting the advancement of medical devices in Latin America. Utilizing the expertise of bioaccess® can further improve the success of these studies, ensuring comprehensive management services from feasibility assessments to post-market follow-up.
Develop Effective Recruitment Strategies for Clinical Trial Participants
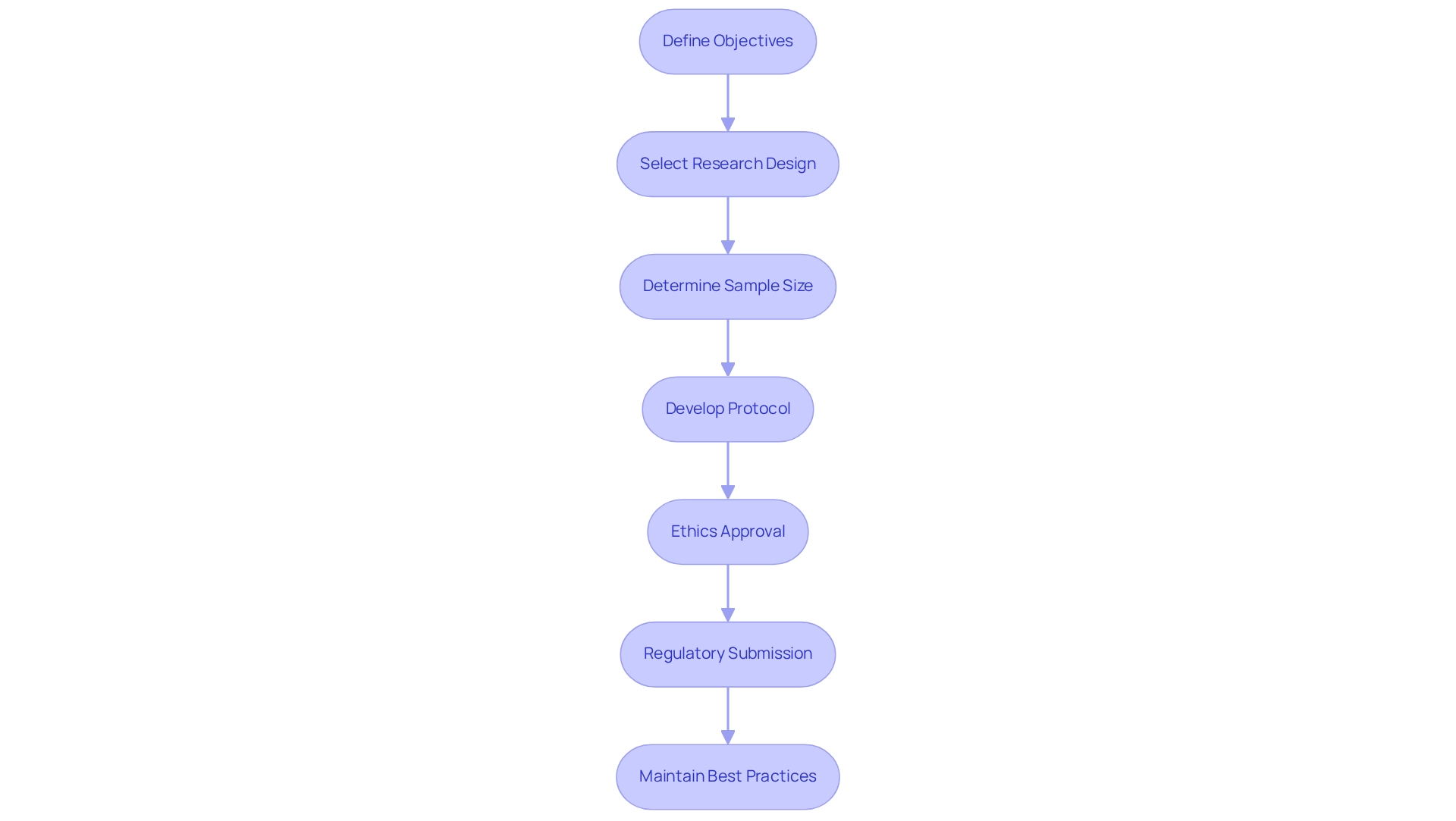
Recruiting participants for clinical studies presents unique challenges; however, implementing effective strategies can significantly enhance enrollment rates. To achieve this, consider the following key approaches:
- Identify Target Population: Clearly define the characteristics of your ideal participants based on the study's inclusion and exclusion criteria. This precision aids in targeting the right demographic.
- Engage Healthcare Providers: Collaboration with local healthcare providers is crucial. Their involvement not only increases awareness about the proceedings but also promotes referrals, leveraging their established trust within the community. This is particularly vital in areas like Barranquilla, where bioaccess™ and Caribbean Health Group are working together to establish the city as a prominent location for clinical research in Latin America, supported by the Colombian Minister of Health.
- Utilize Digital Platforms: Harness the power of social media and online patient communities to reach potential participants. Providing clear, accessible information about the study can clarify the process and stimulate interest.
- Community Outreach: Organizing informational sessions in community centers or hospitals can educate the public about the study and its benefits. This direct engagement fosters a sense of community involvement and trust.
- Incentives: Offering incentives, such as travel reimbursements or health check-ups, can motivate individuals to participate. This approach acknowledges the commitment required from participants and can enhance enrollment rates.
- Acknowledge Challenges: It is essential to recognize the obstacles encountered in participant recruitment for research studies in 2025, such as competition for participants and varying levels of awareness about research initiatives.
By implementing these strategies, research studies can enhance participant recruitment and retention, ultimately leading to more successful outcomes. Notably, recent data indicates that 75 percent of patients expressed a readiness to participate in research studies if informed about the options, underscoring the potential for increased involvement through efficient outreach. Furthermore, the COVID-19 pandemic has expedited the adoption of decentralized trials, demonstrating their feasibility and advantages, which can serve as a model for innovative recruitment strategies. As emphasized by Julio G. Martinez-Clark, aligning with best practices, such as those facilitated by OECD membership, can enhance the effectiveness of recruitment efforts in the region. The knowledge and tailored approach of bioaccess® aim to assist in accelerating medical innovations for firms in the medtech sector, highlighting the significance of efficient recruitment strategies. Additionally, bioaccess® focuses on a range of trials, including Early-Feasibility Trials, First-In-Human Trials, Pilot Trials, Pivotal Trials, and Post-Market Follow-Up Trials, which are essential for the advancement of medical technology and can greatly influence local economies through job creation and enhancements in healthcare.
Implement Post-Market Clinical Follow-Up Studies for Continuous Compliance
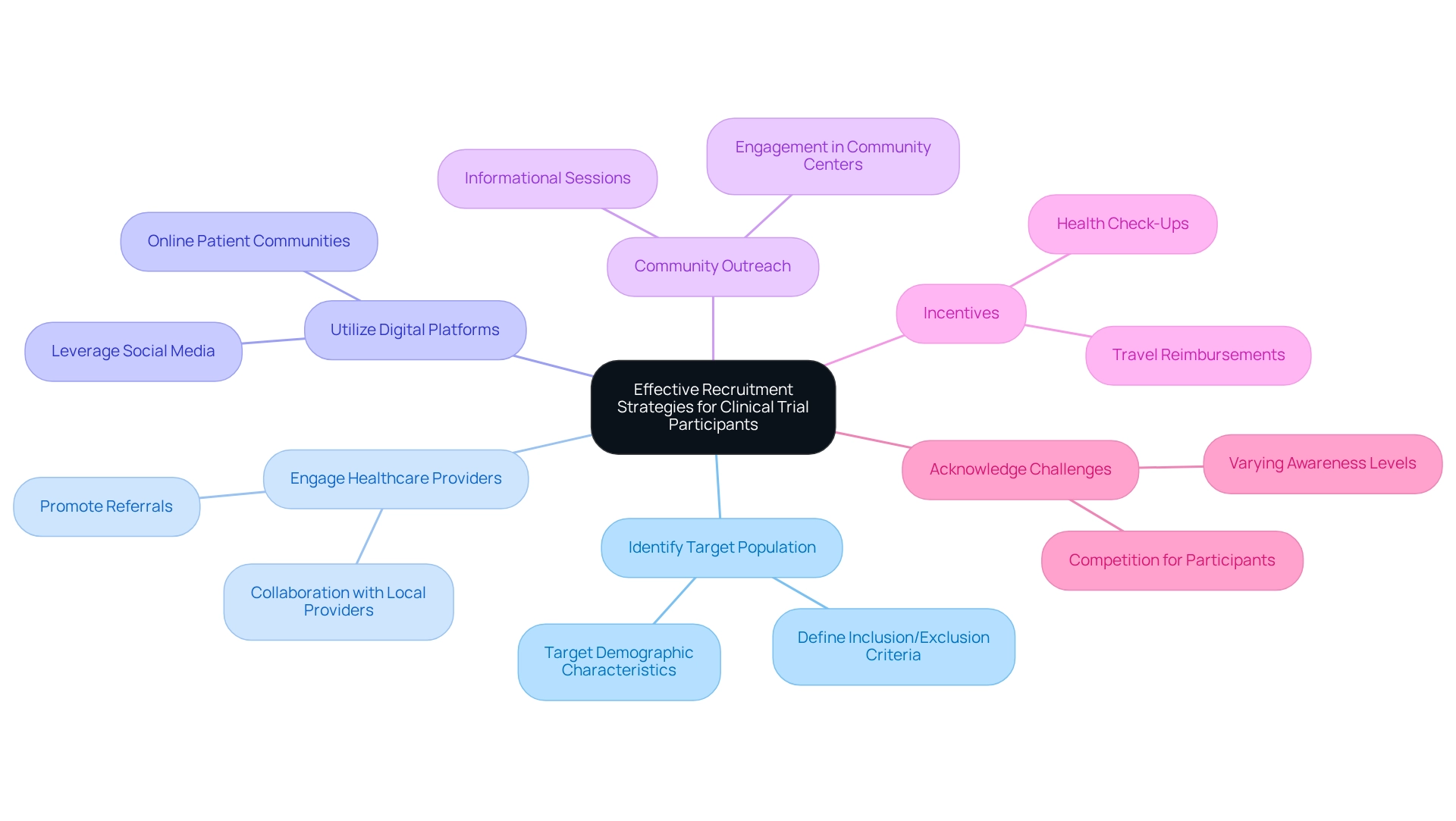
Post-market clinical follow-up (PMCF) evaluations are vital for ensuring the ongoing safety and efficacy of medical instruments. These research efforts not only aid in tracking equipment performance but also play a crucial role in regulatory compliance and risk management. To effectively implement PMCF studies, consider the following key steps:
- Establish a PMCF Plan: Develop a comprehensive PMCF plan that outlines the study's objectives, methodologies, and timelines. This plan must align with regulatory requirements, including the EN 62366 standard related to usability, and address specific safety and efficacy concerns.
- Data Collection: Employ systematic data gathering techniques to accumulate detailed information on equipment performance, including any adverse events. This method ensures that all pertinent data is captured for thorough analysis.
- Examine Information: Regularly review the collected data to identify trends or emerging issues throughout the product's lifecycle. This proactive analysis is essential for timely interventions and risk mitigation.
- Report Findings: Ensure that findings are reported to DINAVISA as required, maintaining transparency and compliance with regulatory expectations. This step is crucial for demonstrating adherence and fostering trust with regulatory bodies.
- Continuous Improvement: Leverage insights gained from PMCF research to inform product enhancements and updates. This iterative process not only improves the safety and efficacy of tools but also aligns with the evolving needs of patients and healthcare providers.
The significance of PMCF evaluations is underscored by recent statistics indicating that regular updates to Clinical Evaluation Reports (CER) based on post-market surveillance data can lead to essential corrective actions, including modifications to product design and labeling. A case analysis titled 'Updating the Clinical Evaluation Report (CER)' illustrates the importance of routinely refreshing the CER based on new information from postmarket surveillance activities, highlighting the necessity for a systematic approach to determine the frequency of updates. Successful PMCF evaluations have shown substantial impacts on equipment safety, reinforcing the need for a structured method of compliance. As Dietmar Falke, a senior consultant in clinical research, notes, 'The continuous assessment of medical instruments through PMCF evaluations is crucial for ensuring their safety and efficacy in the market.' By prioritizing robust PMCF research, medical device manufacturers can ensure their products remain compliant and effectively meet the needs of the healthcare market. With Bioaccess's expertise in managing PMCF studies, manufacturers can navigate the complexities of regulatory compliance and enhance their product's market performance.
Conclusion
Successfully navigating clinical trials for medical devices in Paraguay hinges on a thorough understanding of DINAVISA's regulatory framework. Awareness of the approval processes, ethical guidelines, and international standards is essential for researchers aiming to enhance device efficacy and safety. The recent increase in clinical research interest, particularly due to the COVID-19 pandemic, presents significant opportunities for advancement.
Designing effective clinical trials involves a structured approach that includes:
- Defining clear objectives
- Selecting suitable study designs
- Securing ethical approvals
By following these steps, researchers can create robust trials that contribute to the development of medical technology in Latin America. Additionally, effective recruitment strategies—such as engaging healthcare providers and utilizing digital platforms—are crucial for boosting participant enrollment and retention.
Post-market clinical follow-up studies play a vital role in ongoing compliance and risk management, allowing manufacturers to monitor device performance and address safety concerns promptly. These studies are essential for continuous improvement and ensuring that devices meet regulatory standards.
In summary, the success of clinical trials in Paraguay relies on strategic planning, regulatory compliance, and ethical practices. By leveraging the right insights and partnerships, researchers can navigate this evolving landscape, leading to innovations that enhance healthcare outcomes and fulfill the needs of patients and providers alike.
Frequently Asked Questions
What is required to design clinical trials for medical devices in Paraguay?
Designing clinical trials in Paraguay requires a comprehensive understanding of the regulatory framework established by DINAVISA, which includes familiarity with the research approval process and the submission of extensive documentation outlining the study's objectives, methodologies, and ethical considerations.
What is Resolution 614 and why is it important?
Resolution 614 delineates the procedures for obtaining research study approval in Paraguay, detailing the necessary documentation and anticipated timelines for review. It is important for ensuring that researchers follow the correct processes for approval.
What ethical considerations must be adhered to during clinical trials?
Adherence to ethical standards, particularly regarding informed consent and participant safety throughout the trial, is crucial.
Why is compliance with global standards like Good Clinical Practice (GCP) important?
Compliance with international guidelines such as Good Clinical Practice (GCP) is vital because these standards may influence local regulatory requirements and help ensure the quality and integrity of the research.
What trends have been observed in research submissions in Paraguay?
There has been a growing interest in research involving human participants in Paraguay, with a significant increase in submissions during 2018 and 2019. However, the total number of analyzed submissions by Ethics Review Boards (ERBs) remained below 50 research projects from 2015 to 2020.
How has the COVID-19 pandemic affected medical research in Paraguay?
The COVID-19 pandemic has stimulated growth in medical research opportunities, particularly related to COVID-19 therapies and vaccines, leading to the establishment of recognized ERBs and improved collaboration among healthcare professionals and researchers.
How can partnering with bioaccess® benefit researchers in Paraguay?
Partnering with bioaccess® can enhance the research approval process by providing expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring that all regulatory and compliance aspects are meticulously handled, which can improve timelines and success rates in approvals.




