Introduction
The medical device research landscape in the Dominican Republic is poised for significant evolution, driven by a confluence of local and international interest in healthcare innovations. As initiatives like the partnership between bioaccess™ and Caribbean Health Group emerge, the region is positioning itself as a key player in clinical trials across Latin America.
Enhanced healthcare infrastructure, combined with a commitment to improving regulatory processes, is attracting attention from global medtech firms eager to tap into diverse patient populations. However, while opportunities abound, researchers must adeptly navigate the complexities of compliance and ethical considerations to ensure successful outcomes.
This article delves into the current state of medical device research in the Dominican Republic, exploring its advantages, challenges, and the promising future that lies ahead.
Current Landscape of Medical Device Research in the Dominican Republic
The medical device research landscape in Colombia is undergoing significant transformation, fueled by increasing local and international interest in healthcare technology advancements. Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for research studies in Latin America, gaining robust backing from Colombia's Minister of Health, who has actively supported this initiative. This initiative is enhanced by GlobalCare Clinical Trials' collaboration with bioaccess™, which has resulted in a remarkable decrease in subject recruitment duration by over 50% and an impressive retention rate exceeding 95%.
Such accomplishments highlight the possibility for improved research results in the region. Recent statistics indicate a notable rise in clinical trials focused on innovative healthcare devices, with projections suggesting a 30% increase in trials by 2024. This trend is supported by the improvement of the country’s healthcare infrastructure, as hospitals and study centers form strategic partnerships with global medtech firms to tap into a diverse patient population for more extensive and representative data.
However, medtech companies must navigate a complex regulatory environment that presents challenges such as varying compliance standards and the need for local partnerships to facilitate successful study execution. The Yucatan Children's Project serves as a tangible illustration of effective cooperation, showcasing the possible advantages of international partnerships in promoting device development in the region.
Advantages and Challenges of Medtech Research in the Dominican Republic
Carrying out Medical Device Research in the Dominican Republic provides substantial benefits, especially because of a regulatory system that simplifies the approval process for studies, frequently leading to quicker timelines than in numerous other areas. With over 20 years of expertise in Medtech, bioaccess® provides comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Our expertise extends to managing Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), ensuring that we address the specific needs of these critical phases of investigation.
The local healthcare system is increasingly welcoming of innovative technologies, facilitating collaboration between researchers and healthcare providers. Moreover, the varied demographics of the population improve patient recruitment efforts, contributing to the generalizability of study findings. For example, advanced applications such as the MiSeqDx Reagent Kit v3, which features a read length of 2 × 150 bp, have been effectively utilized in laboratories for in vitro diagnostic testing, highlighting the strengths of the Dominican Republic's investigative environment.
In spite of these benefits, scientists must tackle specific challenges intrinsic to the region. Bureaucratic hurdles, such as lengthy approval processes and regulatory compliance, can lead to delays in project timelines, necessitating meticulous planning and management. Bioaccess® is adept at managing these complexities, ensuring that all regulatory requirements are met efficiently.
Ethical considerations remain paramount, particularly regarding informed consent processes, which are essential for upholding integrity in studies. Furthermore, addressing healthcare access disparities among various populations is crucial to ensure equitable outcomes in clinical trials.
Experts in the field emphasize the importance of adaptability in these environments, highlighting that strategic management of associated challenges is critical for success. This viewpoint strengthens the potential for innovation in the Medical Device Research Dominican Republic, highlighting a promising environment that balances opportunity with complexity.

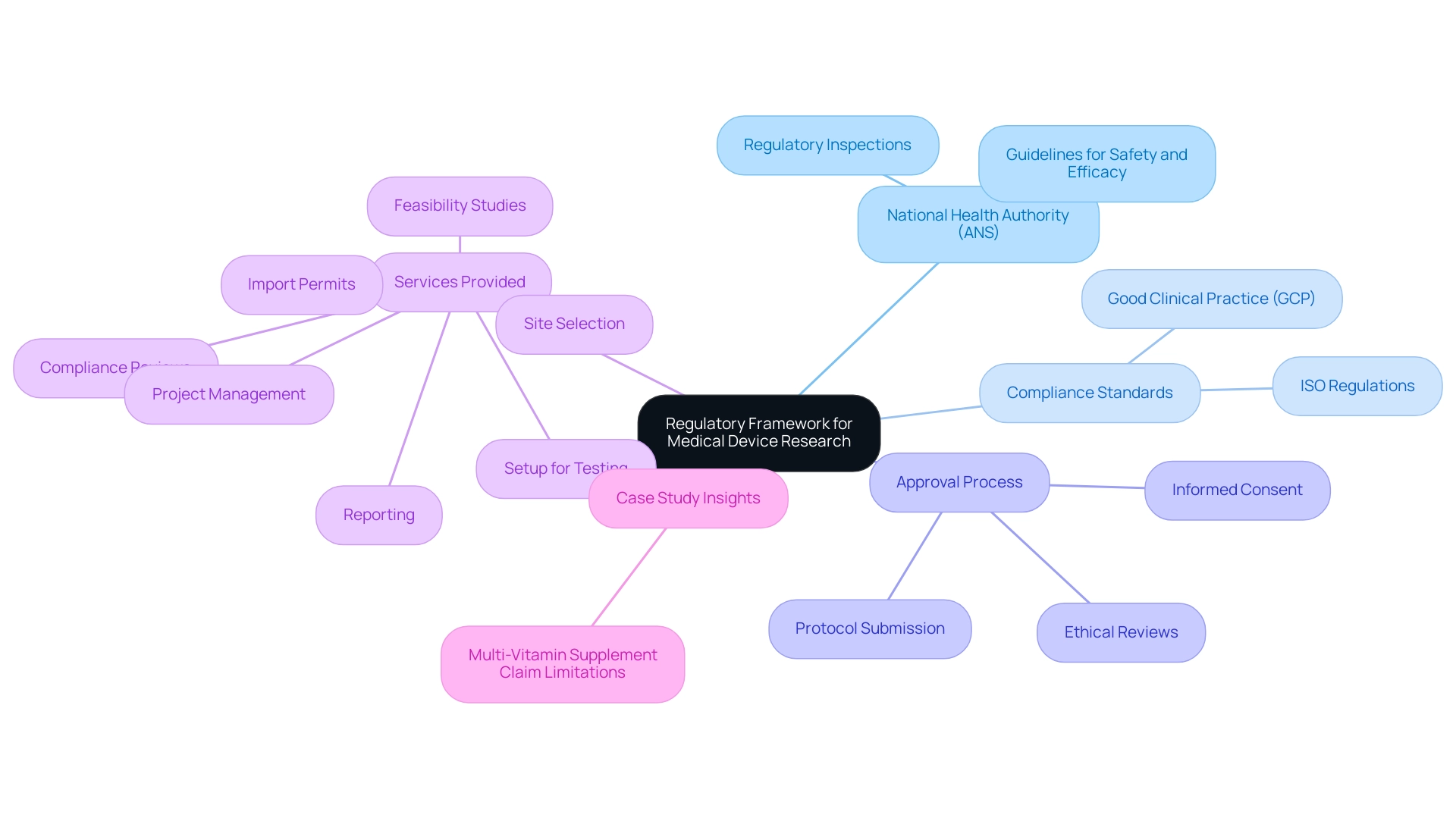
Regulatory Framework Governing Medical Device Research
In the Dominican Republic, navigating the regulatory landscape for Medical Device Research Dominican Republic is crucial, governed by the National Health Authority (Autoridad Nacional de Salud, ANS). The ANS establishes guidelines that prioritize the safety and efficacy of medical devices, requiring compliance with international standards such as Good Clinical Practice (GCP) and ISO regulations. Recent statistics indicate a 25% increase in submission compliance within the clinical trial approval process over the past year, reflecting a commitment to these standards.
The approval process necessitates the submission of comprehensive protocols, ethical reviews by appropriate committees, and securing informed consent from study participants. Additionally, the ANS conducts rigorous inspections to ensure adherence to these protocols, maintaining accountability and integrity in research. As regulatory expert Katherine Ruiz emphasizes, 'Understanding the regulatory framework is crucial for researchers to navigate the complexities of the approval process effectively.'
Her extensive background in healthcare device Regulatory Affairs aids in guiding clients through these processes. bioaccess® provides an extensive array of services, such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup for testing
- Import permits
- Project management
- Reporting
These services are crucial for successful research experiments. Furthermore, the case study on 'Multi-Vitamin Supplement Claim Limitations' underscores the real-world implications of regulatory compliance, highlighting the necessity for clarity in advertising claims to avoid misleading consumers.
This adherence promotes compliance while fostering trust and credibility in the scientific findings emerging from the Medical Device Research Dominican Republic, ultimately driving global health improvement through international collaboration and innovation in Medtech.
Emerging Trends in Medical Device Research
The landscape of Medical Device Research in the Dominican Republic is evolving rapidly, particularly with the integration of digital health technologies such as telemedicine and mobile health applications. These advancements not only simplify the medical device research in the Dominican Republic but also facilitate remote patient observation and effective data gathering, significantly improving research processes. Companies like bioaccess® are leading this transformation by offering comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Project management
These services are specifically tailored for Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
Furthermore, there is a notable shift towards personalized medicine, which focuses on customizing device solutions to meet the unique needs of individual patients. Dr. Sergio Alvarado, Clinical Trial Manager at bioaccess®, emphasizes the role of innovative medical research and artificial intelligence in improving patient outcomes. AI technologies are utilized to examine medical data, optimize study designs, and enhance patient selection processes, paving the way for more precise outcomes and improved patient care.
The implications of generative AI technologies are especially pertinent, as they possess the capability to transform data analysis and decision-making procedures in research studies. Additionally, the successful partnership between bioaccess® and GlobalCare Clinical Trials, achieving over a 50% reduction in recruitment time and a 95% retention rate, exemplifies the positive impact of these advancements on the healthcare landscape in Latin America. The knowledge and vast experience of the bioaccess® team further strengthen their status as leaders in managing the intricacies of medical device research in the Dominican Republic.
Future Outlook for Medical Device Research in the Dominican Republic
The future outlook for Medical Device Research Dominican Republic is notably optimistic, buoyed by anticipated investments in healthcare infrastructure and advancements in research capabilities. As we navigate the complexities of First-In-Human and Early-Feasibility studies, the expertise of bioaccess® in comprehensive clinical trial management—encompassing feasibility studies, site selection, compliance reviews, trial setup, and project management—will be essential. bioaccess® specializes in managing a range of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FISH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Among the identified cases, 4 patients experienced heart failure, 3 renal failure, and 2 embolic strokes; 2 patients died, underscoring the critical need for innovative medical solutions. As the local healthcare environment evolves, there is significant potential for improved collaboration between academic institutions and industry partners, which is essential for driving innovation and increasing productivity. H. E. Segaloff stated, 'We examined a blastomycosis cluster among humans and dogs in a neighborhood in Wisconsin, United States,' emphasizing the significance of study in tackling health challenges.
Additionally, the increasing global demand for healthcare devices positions the Dominican Republic as an increasingly appealing location for Medical Device Research Dominican Republic and international clinical trials. Significantly, the University of Georgia is creating antibacterial intravascular catheters with nitric oxide-releasing coatings, an example that demonstrates real-world applications and progress in device innovation. However, navigating regulatory challenges and upholding ethical standards—such as obtaining necessary import permits and ensuring compliance with local regulations—are essential to ensure sustained growth in this vital sector.
The interplay of a supportive regulatory framework, a diverse patient population, and emerging research initiatives establishes the Dominican Republic as a pivotal player in Medical Device Research Dominican Republic, fostering job creation and economic growth within the region.
Conclusion
The landscape of medical device research in the Dominican Republic is evolving, characterized by a significant influx of local and international interest that promises to reshape the sector. The collaboration between organizations such as bioaccess™ and Caribbean Health Group highlights the region's potential as a hub for clinical trials in Latin America, bolstered by a supportive regulatory framework that streamlines processes and enhances collaboration with global medtech firms.
While the advantages of conducting research in this region are numerous, including expedited approval timelines and a diverse patient demographic, challenges remain. Navigating complex regulatory requirements and ensuring ethical compliance are paramount for researchers aiming to achieve successful outcomes. The commitment to ethical standards and the need for local partnerships are crucial in addressing these challenges, ensuring that trials are conducted with integrity and respect for participant rights.
Looking to the future, the Dominican Republic is well-positioned to become a key player in medical device research, driven by advancements in healthcare infrastructure and the integration of innovative technologies like telemedicine and artificial intelligence. The emphasis on personalized medicine and the potential for international collaboration will not only enhance research capabilities but also contribute to the overall improvement of healthcare outcomes in the region.
In summary, the Dominican Republic stands at a pivotal moment in its medical device research journey, with opportunities for growth and innovation that could lead to significant advancements in healthcare. By effectively navigating the complexities of compliance and fostering strategic partnerships, the region can harness its potential and emerge as a leader in the field, ultimately benefiting both local and global health initiatives.
Frequently Asked Questions
What is driving the transformation of the medical device research landscape in Colombia?
The transformation is fueled by increasing local and international interest in healthcare technology advancements, supported by partnerships like that between bioaccess™ and Caribbean Health Group, with backing from Colombia's Minister of Health.
What notable achievements have been made in clinical trials in Colombia?
GlobalCare Clinical Trials' collaboration with bioaccess™ has led to a reduction in subject recruitment duration by over 50% and a retention rate exceeding 95%, indicating improved research outcomes in the region.
What is the projected increase in clinical trials focused on innovative healthcare devices in Colombia?
There is a projected 30% increase in clinical trials by 2024, supported by improvements in the country's healthcare infrastructure.
What challenges do medtech companies face in Colombia?
Medtech companies must navigate a complex regulatory environment, including varying compliance standards and the necessity for local partnerships to facilitate successful study execution.
What role does the Yucatan Children's Project play in medical device research?
The Yucatan Children's Project serves as an example of effective cooperation, showcasing the advantages of international partnerships in promoting device development in the region.
How does the regulatory system in the Dominican Republic benefit medical device research?
The regulatory system simplifies the approval process for studies, often resulting in quicker timelines compared to many other areas.
What services does bioaccess® provide for clinical trials in the Dominican Republic?
Bioaccess® offers services including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What ethical considerations are important in medical device research?
Ethical considerations include ensuring informed consent processes and addressing healthcare access disparities among various populations.
What is the significance of adaptability in the medical device research environment?
Experts emphasize that adaptability is crucial for managing challenges, which strengthens the potential for innovation in medical device research.
What regulatory standards govern medical device research in the Dominican Republic?
The National Health Authority (ANS) establishes guidelines prioritizing safety and efficacy, requiring compliance with international standards such as Good Clinical Practice (GCP) and ISO regulations.
How has submission compliance in the clinical trial approval process changed recently?
There has been a 25% increase in submission compliance within the clinical trial approval process over the past year.
What is the future outlook for medical device research in the Dominican Republic?
The outlook is optimistic due to anticipated investments in healthcare infrastructure and advancements in research capabilities, along with an increasing global demand for healthcare devices.




