Introduction
Navigating the complex landscape of medical device trials in Peru requires a comprehensive understanding of the regulatory framework and the procedural steps involved. With oversight from the National Institute of Health (INS) and the Ministry of Health (MINSA), researchers must adhere to specific laws and guidelines to ensure the safety and efficacy of their trials. This article delves into the essential regulations governing medical device trials, the meticulous process for conducting these studies, and the critical importance of ethical considerations such as:
- Informed consent
- Adverse event monitoring
By leveraging expert guidance and adhering to best practices, researchers can successfully navigate the intricacies of clinical trials, ultimately contributing to advancements in medical technology and public health.
Understanding the Regulatory Framework for Medical Device Trials in Peru
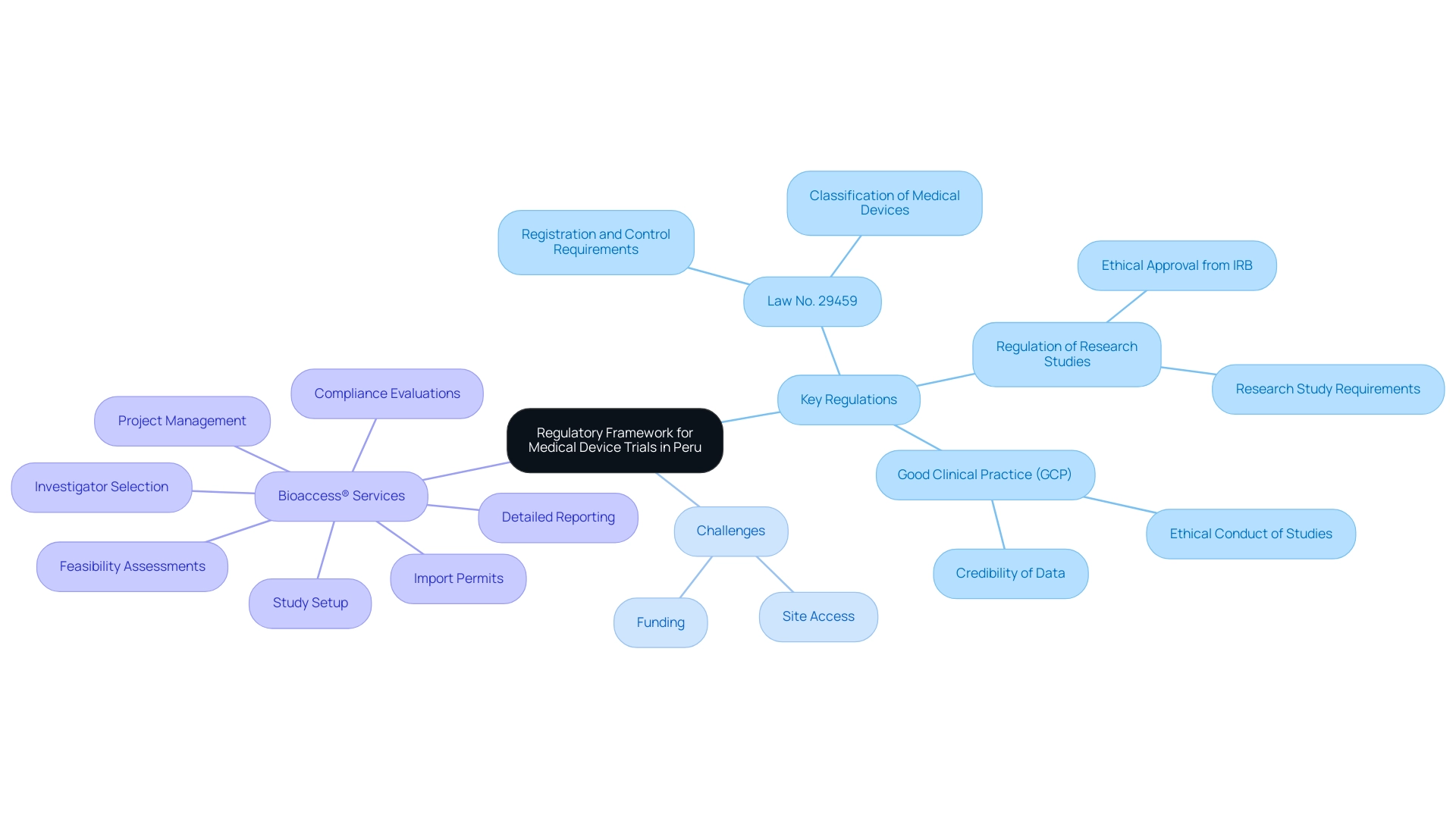
In Peru, the assessments of medical devices are part of the Peru Medical Device Trials governed by a specific regulatory framework established by the National Institute of Health (INS) and the Ministry of Health (MINSA). Familiarize yourself with the following key regulations:
- Law No. 29459: This law regulates the registration and control of medical devices. It is essential to understand the classification of medical devices and the corresponding requirements for each category.
- Regulation of Research Studies: The regulation outlines the requirements for conducting research studies, including the necessity of obtaining ethical approval from an Institutional Review Board (IRB).
- Good Clinical Practice (GCP): Compliance with GCP guidelines is mandatory. These guidelines ensure that studies are conducted ethically and that the data collected is credible.
Given the complex regulatory environment and potential challenges such as securing site access and funding, partnering with experts like bioaccess® can significantly streamline the research process. Their thorough research study management services encompass:
- Feasibility assessments
- Investigator selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Detailed reporting
It is essential to highlight that obtaining ethical approvals and import permits is a vital step in the research procedure.
Before starting a test, ensure that all necessary permits and approvals are obtained from the relevant authorities. This foundational understanding of the regulatory landscape, along with the specific services provided by bioaccess®, will help mitigate potential legal issues and enhance the success of your clinical studies.
Step-by-Step Process for Conducting Medical Device Trials
Conducting medical device evaluations in Peru involves several key steps:
-
Pre-Trial Preparation:
- Identify the medical device and its intended use.
- Develop a detailed study protocol outlining objectives, methodology, and statistical analysis plans, ensuring compliance with local regulations and GCP guidelines.
- bioaccess® provides expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up Studies, ensuring a robust preparation phase backed by over 20 years of experience in Medtech.
-
Ethics Approval:
- Submit the study protocol to an Institutional Review Board (IRB) for ethical review and approval.
- Address any feedback or required modifications from the IRB to ensure compliance.
-
Regulatory Submission:
- Prepare and submit the necessary documentation to the INS, including the study protocol, informed consent forms, and any additional required information, leveraging bioaccess®'s regulatory expertise for smooth navigation through the approval process, which includes comprehensive guidance on local regulatory requirements.
- Wait for approval before commencing the trial.
-
Recruitment of Participants:
- Develop a recruitment strategy to enroll eligible participants, ensuring informed consent is obtained from all subjects.
- Consider demographic factors and inclusion/exclusion criteria to ensure a representative sample, supported by bioaccess®'s experience in feasibility studies and site selection.
-
Trial Execution:
- Conduct the trial according to the approved protocol, ensuring adherence to GCP.
- Monitor participant safety and data integrity throughout the trial, with comprehensive project management services offered by bioaccess®, including regular site monitoring and data verification to ensure compliance and quality.
-
Data Analysis and Reporting:
- Analyze the collected data following the predefined statistical methods.
- Prepare a final report summarizing the trial outcomes, including any adverse events or deviations from the protocol, with assistance from bioaccess® in reporting best practices.
-
Post-Trial Activities:
- Submit the final report to the INS and IRB.
- Consider publishing the results in a peer-reviewed journal to contribute to the medical community. This aligns with the commitment of professionals like Juan Cuya, MD, and Katherine Ruiz to advance knowledge in medical technology and public health. By following these steps meticulously and leveraging the comprehensive clinical trial management services from bioaccess®, researchers can effectively navigate the complexities of conducting Peru Medical Device Trials.
Ensuring Informed Consent from Participants
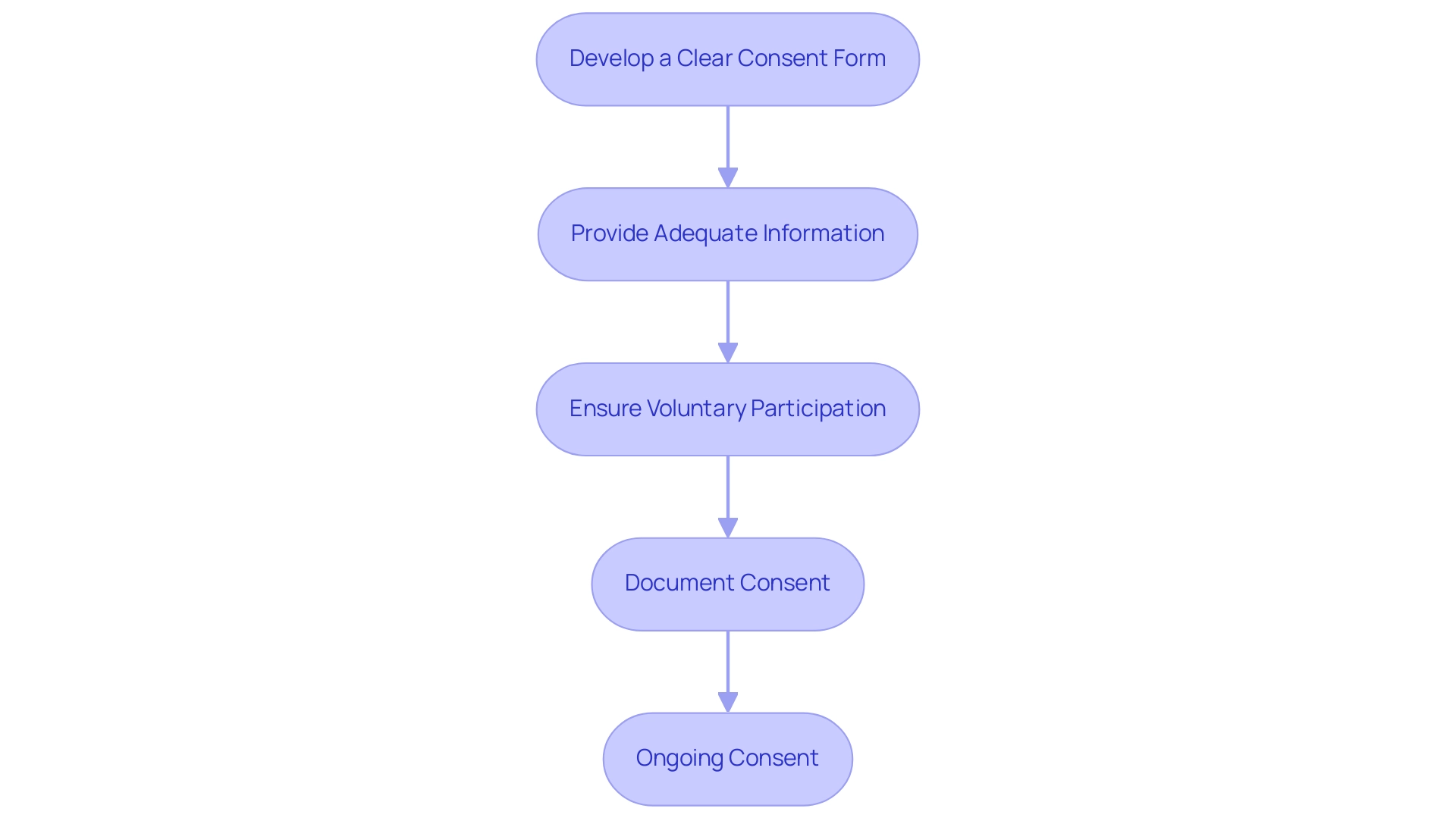
To ensure informed consent from those involved, follow these steps:
-
Develop a Clear Consent Form:
- Create a consent form that is easy to understand, avoiding technical jargon. It should clearly outline the study's purpose, procedures, potential risks, and benefits.
-
Provide Adequate Information:
- Before obtaining consent, provide participants with all necessary information about the study. Allow them sufficient time to ask questions and consider their participation.
-
Ensure Voluntary Participation:
- Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
-
Document Consent:
- Obtain written consent from each participant and keep a signed copy on file. This documentation is crucial for compliance and ethical accountability.
-
Ongoing Consent:
- During the trial, continue to inform participants about any new findings or changes that may affect their willingness to participate, ensuring that consent remains valid throughout the study.
By adhering to these guidelines, researchers can uphold ethical standards and foster trust with participants, which is essential for the success of any clinical trial.
Monitoring and Reporting Adverse Events
Effectively monitoring and reporting negative occurrences during medical device studies is crucial for ensuring participant safety and maintaining the integrity of the research process. To achieve this, researchers should follow these key steps:
-
Establish a Monitoring Plan:
- Create a comprehensive plan that outlines how adverse events will be monitored, reported, and managed throughout the trial.
This ensures a systematic approach to handling potential issues, aligned with the comprehensive clinical trial management services offered, including feasibility studies, compliance reviews, trial setup, and project management.
-
Train Study Staff:
- It is vital to train all study personnel to recognize and promptly report adverse events. Understanding the definitions of adverse events and serious adverse events according to regulatory guidelines fosters a culture of vigilance.
As Donna Angel Carico from Atrium Health Wake Forest Baptist emphasizes,
A good idea would be to have frequent meetings to make sure Adverse Event reporting is a process that everyone is familiar with so that no reportable event goes unreported.
This training should include insights into the regulatory environment established by INVIMA, the Colombian authority overseeing medical device compliance.
-
Document Adverse Events:
- Keep detailed records of all adverse events, including their nature, severity, and outcomes.
This documentation is not only essential for regulatory reporting to authorities like INVIMA but also critical for assessing the safety profile of the medical device.
-
Report to Authorities:
- Comply with local regulations regarding the reporting of adverse events to authorities such as the INS and INVIMA. Timely and precise reporting is vital for safety and fulfilling regulatory obligations, reflecting the importance of adherence to local health standards.
-
Review and Adjust Protocols:
- Regularly analyze data related to adverse events to detect trends or emerging safety concerns. For example, during a Phase 2 study, an individual experienced a severe allergic reaction, prompting immediate protocol amendments. These amendments not only enhanced safety for those involved but also improved future study designs, showcasing the necessity of vigilance in managing adverse events.
Be prepared to adjust study protocols as necessary to improve participant safety, showing adaptability in reaction to real-time data.
By applying this strong monitoring and reporting framework, researchers can greatly improve participant safety while maintaining the integrity of research studies. Recent case studies underscore the necessity of proactive measures in managing adverse events, further emphasizing the importance of a comprehensive approach to monitoring and reporting, as exemplified by the expertise of regulatory professionals like Katherine Ruiz. Additionally, the systematic processes involved in setup and compliance reviews play a critical role in ensuring that adverse event monitoring aligns with the comprehensive services provided, reinforcing the overall effectiveness of clinical research management.
Data Management and Integrity
To ensure information management and integrity during medical device evaluations, follow these practices:
-
Develop a Data Management Plan:
- Create a comprehensive plan that outlines how data will be collected, stored, and analyzed. This plan should address information security measures and compliance with regulatory requirements, reflecting the thorough approach taken by our clinical trial management services, including feasibility studies and site selection.
-
Use Electronic Data Capture (EDC) Systems:
- Implement EDC systems to streamline data collection and minimize errors. Ensure that the system is validated and compliant with relevant regulations, as advised by our Regulatory Affairs expert, Katherine Ruiz, who has extensive experience in ensuring compliance with local regulations in Colombia.
-
Regular Data Monitoring:
- Conduct regular audits of the data to identify inconsistencies or errors. This monitoring should take place throughout the experiment to ensure continuous information quality, in accordance with our dedication to strict project management and reporting, including serious and non-serious adverse events.
-
Trial Setup and Approval Processes:
- Ensure that all trial setup and approval processes, including ethics committee and health ministry approvals, are meticulously followed to maintain compliance and integrity in the trial.
-
Train Staff on Data Handling:
- Provide training to all staff involved in data management to ensure they understand the importance of data integrity and the proper handling procedures, supported by our expert guidance in clinical trial processes.
-
Maintain Documentation:
- Keep thorough documentation of all data management processes, including data entry, changes, and audits. This documentation is crucial for compliance and for future reference during audits, ensuring alignment with best practices in clinical trials.
By adhering to these data management practices and leveraging our comprehensive clinical trial management services, which include feasibility studies, site selection, and thorough reporting, led by experts like Katherine Ruiz, researchers can enhance the credibility of their trial results and ensure compliance with regulatory standards.
Conclusion
Navigating the landscape of medical device trials in Peru is a multifaceted endeavor that demands a thorough understanding of regulatory frameworks, procedural steps, and ethical considerations. The pivotal role of the National Institute of Health (INS) and the Ministry of Health (MINSA) in overseeing these trials cannot be overstated. Familiarity with key regulations, such as Law No. 29459 and Good Clinical Practice (GCP) guidelines, forms the foundation for conducting compliant and successful trials.
The step-by-step process outlined for conducting these trials emphasizes the importance of:
- Meticulous preparation
- Ethical approvals
- Participant recruitment
By adhering to established protocols, researchers can ensure the safety and well-being of participants while enhancing the integrity of the trial data. Moreover, the commitment to informed consent and the robust monitoring of adverse events are critical components that reinforce ethical standards and foster trust among participants.
Ultimately, the effective management of data integrity and compliance throughout the trial process is essential for achieving credible outcomes. Leveraging expert guidance from specialized clinical trial management services can streamline these complex processes, helping researchers navigate potential challenges and contribute meaningfully to advancements in medical technology and public health. By prioritizing these elements, the medical research community in Peru can continue to progress, ensuring that innovative medical devices are developed with the utmost regard for participant safety and regulatory compliance.
Frequently Asked Questions
What regulatory framework governs medical device assessments in Peru?
Medical device assessments in Peru are governed by the Peru Medical Device Trials, regulated by the National Institute of Health (INS) and the Ministry of Health (MINSA).
What is Law No. 29459?
Law No. 29459 regulates the registration and control of medical devices in Peru, outlining the classification of medical devices and the requirements for each category.
What are the requirements for conducting research studies in Peru?
Research studies must obtain ethical approval from an Institutional Review Board (IRB) and comply with Good Clinical Practice (GCP) guidelines.
How can partnering with bioaccess® help in the research process?
Partnering with bioaccess® can streamline the research process by providing services such as feasibility assessments, investigator selection, compliance evaluations, study setup, import permits, project management, and detailed reporting.
What are the key steps in conducting medical device evaluations in Peru?
The key steps include pre-trial preparation, ethics approval, regulatory submission, recruitment of participants, trial execution, data analysis and reporting, and post-trial activities.
What is involved in the pre-trial preparation phase?
Pre-trial preparation involves identifying the medical device, developing a detailed study protocol, and ensuring compliance with local regulations and GCP guidelines.
What is the process for obtaining ethics approval?
The study protocol must be submitted to an IRB for ethical review and approval, and any feedback or modifications required by the IRB must be addressed.
What documentation is needed for regulatory submission?
Necessary documentation includes the study protocol, informed consent forms, and any additional required information to be submitted to the INS.
How should participant recruitment be conducted?
A recruitment strategy should be developed to enroll eligible participants, ensuring informed consent is obtained and considering demographic factors and inclusion/exclusion criteria.
What steps should be taken during trial execution?
The trial must be conducted according to the approved protocol, ensuring adherence to GCP, monitoring participant safety, and maintaining data integrity.
What is the importance of data analysis and reporting?
Data analysis involves analyzing collected data according to predefined statistical methods and preparing a final report summarizing trial outcomes, which may include adverse events.
What practices should be followed to ensure informed consent?
Researchers should develop a clear consent form, provide adequate information, ensure voluntary participation, document consent, and maintain ongoing consent throughout the trial.
How should adverse events be monitored and reported during studies?
Establish a monitoring plan, train study staff, document adverse events, report to authorities, and regularly review protocols to ensure participant safety and regulatory compliance.
What data management practices should be implemented during evaluations?
Develop a data management plan, use electronic data capture systems, conduct regular data monitoring, ensure compliance with approval processes, train staff on data handling, and maintain thorough documentation.




