Overview
This article outlines the crucial steps for effectively designing trials for wearable devices in Peru, highlighting the significance of:
- Understanding regulatory requirements
- Establishing clear objectives
- Implementing robust data management protocols
It elaborates on the regulatory landscape governed by the Peruvian National Institute of Health, the necessity of informed consent, and the imperative for structured data collection and analysis processes. These elements are essential to ensure ethical compliance and enhance the credibility of trial outcomes.
Introduction
Navigating the world of wearable technology trials presents a complex endeavor, particularly within the intricate regulatory landscape of Peru. As health tech advances rapidly, comprehending the legal framework surrounding clinical research becomes crucial for success. The Peruvian National Institute of Health plays a vital role in trial approvals, emphasizing the importance of informed consent and data protection. For researchers aiming to innovate responsibly, the stakes are high. Organizations must strive to design effective and compliant trials through a structured approach that incorporates:
- Clear objectives
- Robust data management
- Thorough analysis
This article explores the critical components that ensure successful wearable device trials, offering insights to streamline the process and enhance outcomes in the ever-evolving healthcare landscape.
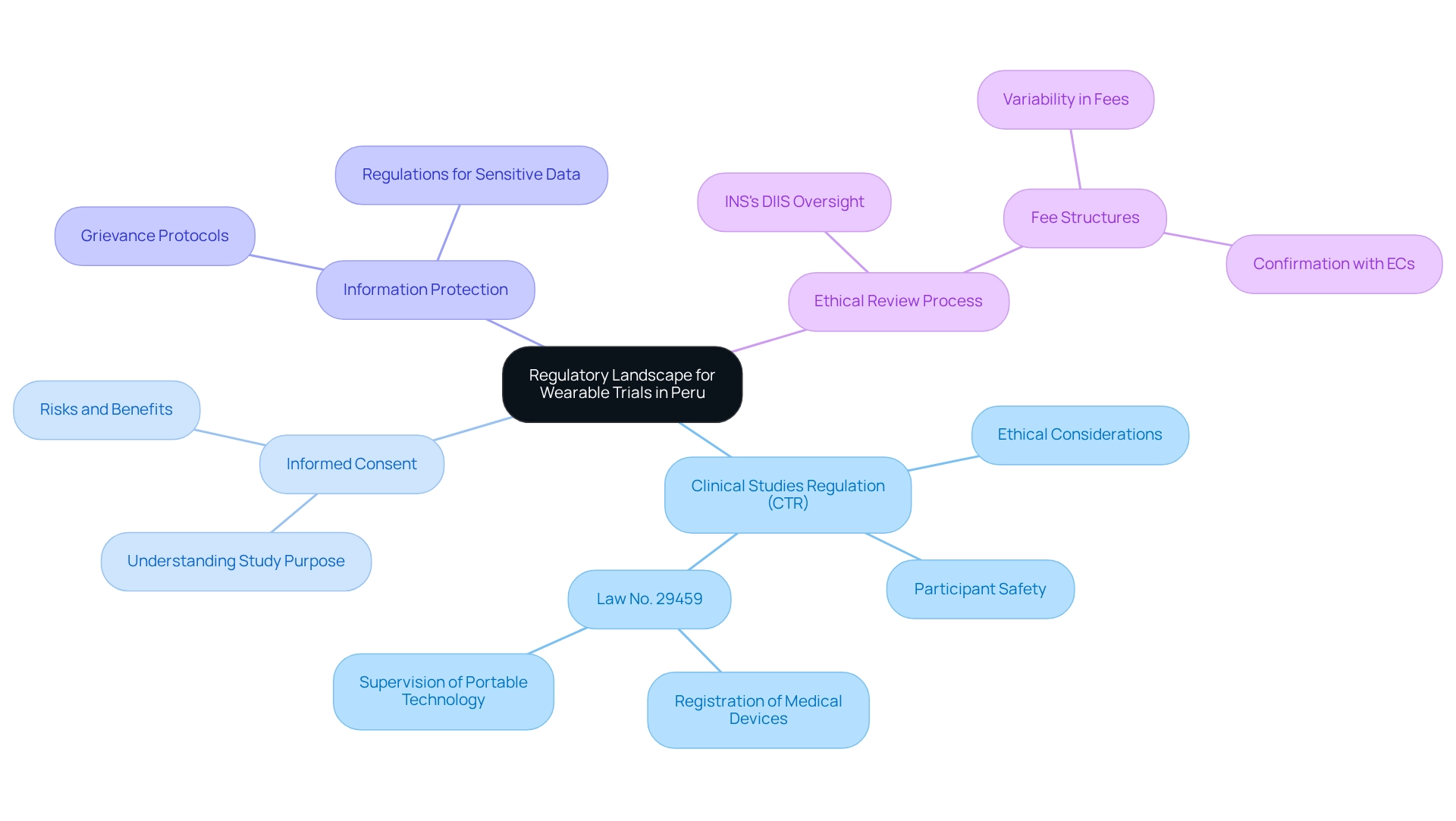
Understand the Regulatory Landscape for Wearable Trials in Peru
Designing trials for wearables in Peru requires effectively navigating the regulatory framework governing clinical research. Begin by reviewing the guidelines established by the Peruvian National Institute of Health (INS), which oversees clinical study approvals and compliance. Key regulations include:
- Clinical Studies Regulation (CTR): This regulation outlines the requirements for conducting clinical studies, emphasizing ethical considerations and participant safety.
Law No. 29459: This law regulates the registration and supervision of medical devices, particularly relevant for portable technology. - Informed Consent: It is crucial that all participants provide informed consent, fully understanding the study's purpose, risks, and benefits.
Information Protection: Familiarity with information protection regulations is vital to secure participant details, especially when utilizing wearable devices that collect sensitive health information. bioaccess® is committed to maintaining information security and client confidence, addressing any issues through established grievance and data protection protocols.
Furthermore, the INS's DIIS supervises the ethical review process for clinical studies, highlighting the importance of ethical adherence in your study design. Understanding the fee structures related to Institutional Ethics Committee evaluations can provide valuable insights into potential expenses and fluctuations in the ethics review process, which is crucial for designing trials for wearables in Peru, enhancing your study design and mitigating potential compliance challenges. For further insights, refer to resources such as the ClinRegs database and the official INS website, which offer comprehensive information on the regulatory environment in Peru. Leveraging the expertise of bioaccess®, specializing in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Post-Market Clinical Follow-Up Studies (PMCF), can further streamline your clinical study processes, ensuring a successful transition from pilot study to commercialization.
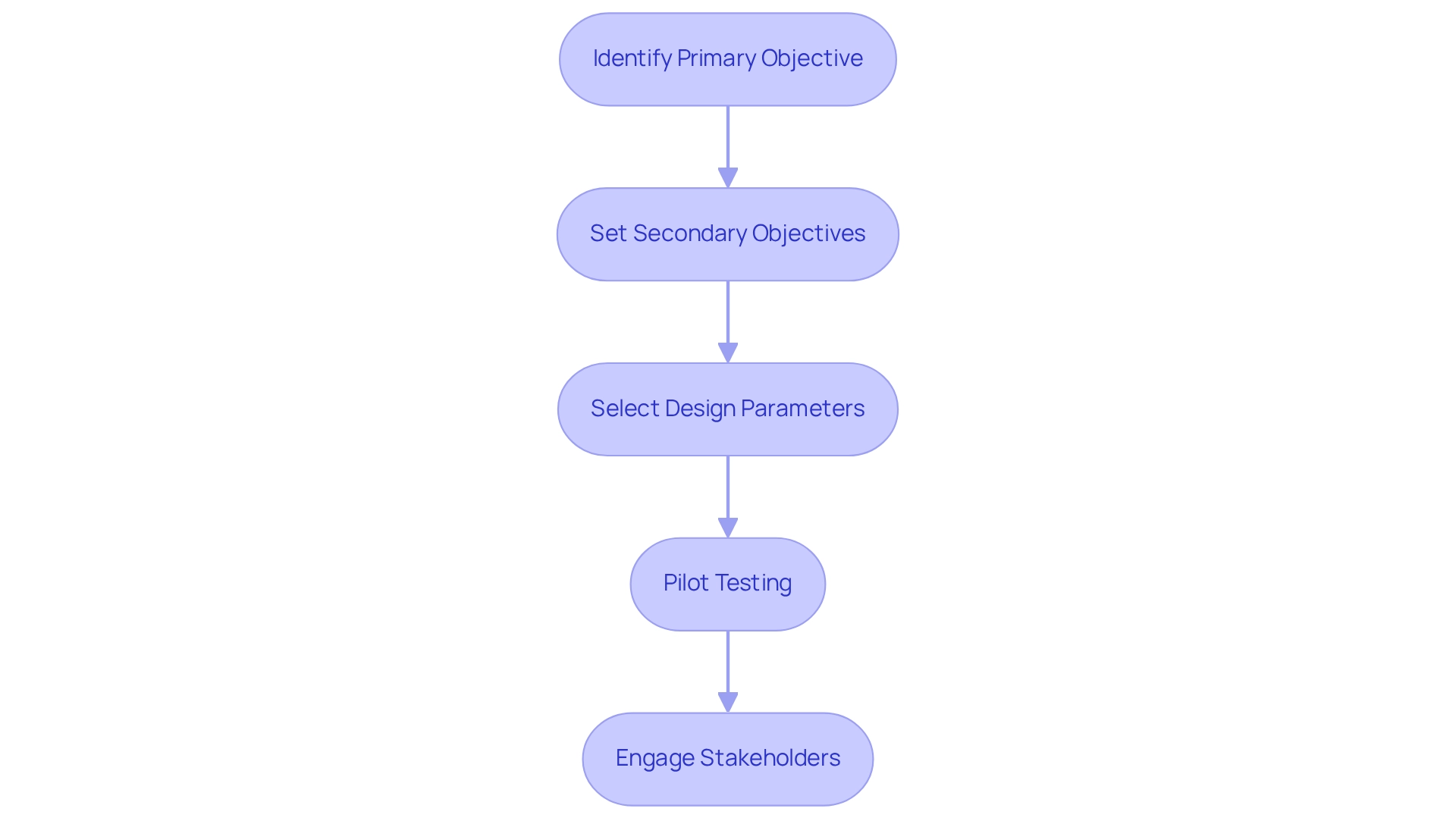
Define Objectives and Design Parameters for Wearable Trials
Establishing clear goals is essential when designing trials for wearables in Peru. Here are essential steps to guide the process:
- Identify the Primary Objective: Determine the main question your trial aims to address. For instance, are you assessing the effectiveness of a device in monitoring heart rate variability?
- Set Secondary Objectives: Consider additional goals such as evaluating user compliance, device comfort, or the device's impact on lifestyle changes.
- Select Design Parameters: Choose parameters that align with your objectives, including the type of wearable device, duration of the research, and demographic characteristics of the population.
- Pilot Testing: Implement a pilot project to refine your objectives and parameters. This initial stage aids in revealing possible problems in the study design, permitting essential modifications before initiating the comprehensive research. Significantly, the guidance document was tested by senior statisticians across five studies in January 2016, highlighting the importance of a structured approach in study design. Leveraging the expertise of bioaccess, which specializes in managing Early-Feasibility and First-In-Human Studies, can enhance the effectiveness of your pilot testing phase.
- Engage Stakeholders: Collaborate with healthcare professionals, regulatory bodies, and potential participants to ensure that your objectives are both realistic and relevant. As Carrol Gamble, PhD, mentions, "Leading organizations and funding bodies openly endorse data sharing as best practice for clinical studies," emphasizing the significance of collaboration and transparency in the research process.
By following these steps, you can create a strong structure for your wearable test, which is essential when considering designing trials for wearables in Peru, significantly enhancing the likelihood of achieving meaningful and impactful results. Successful experiments often combine quantitative and qualitative methods, enhancing patient identification and engagement. Furthermore, compliance with ethical and regulatory standards, including informed consent and participant safety, not only protects participant rights but also bolsters the credibility of your trial outcomes, fostering trust in the research process. For example, adhering to ethical guidelines is essential, as shown in the case analysis titled 'Following Ethical and Regulatory Standards,' which demonstrates how compliance improves credibility and trust in research results. Additionally, remember that formal approval of the SAP from the study sponsor or steering committee is required before distribution to stakeholders, ensuring that all procedural steps are followed.
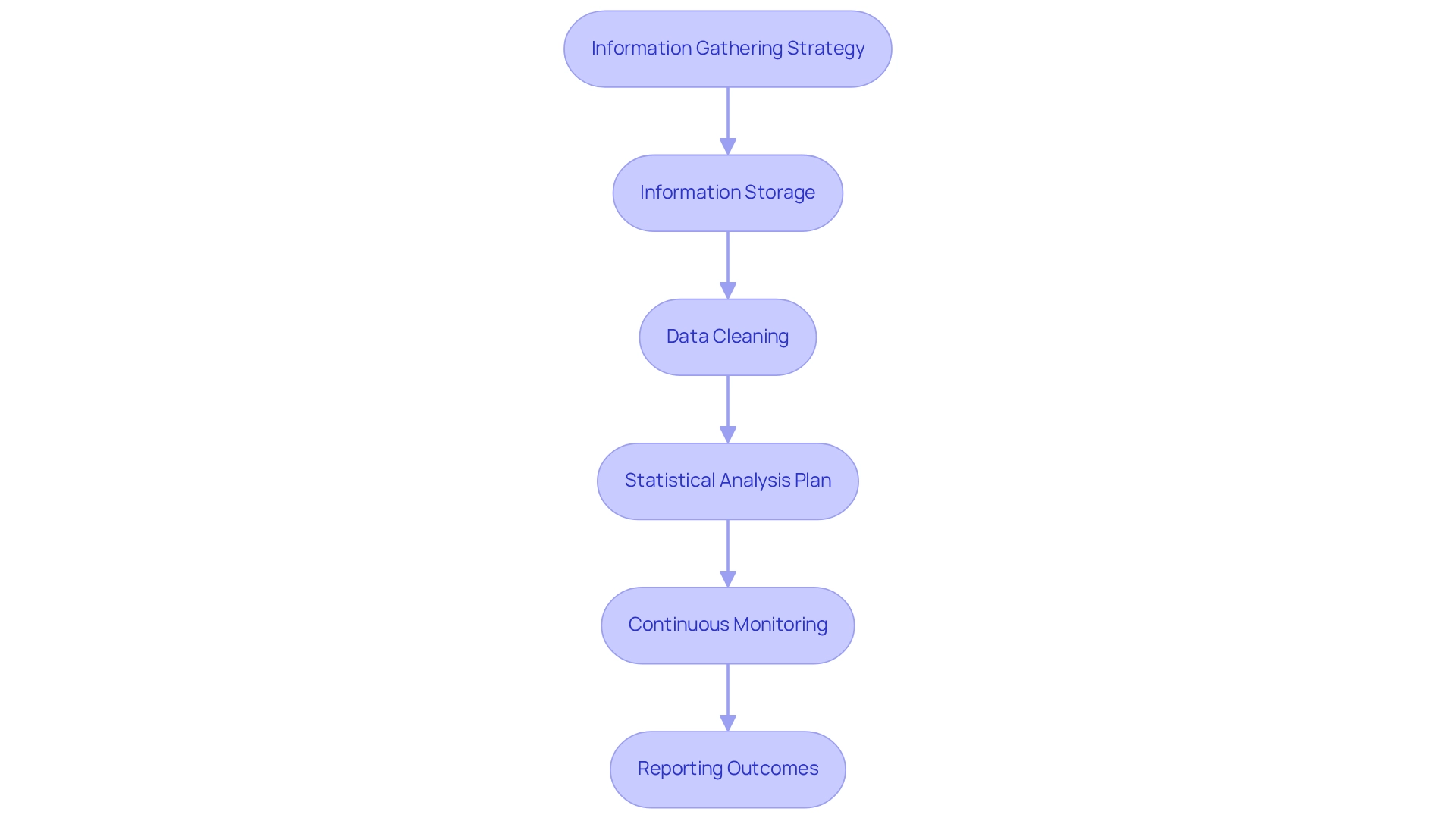
Implement Data Management and Analysis Protocols
To effectively manage and analyze data, designing trials for wearables in Peru requires adherence to the following protocols:
- Information Gathering Strategy: Develop a robust plan for collecting information from wearable devices, ensuring that all devices are properly calibrated and functioning before the trial begins. Acknowledge potential biases and sample size limitations that may affect the integrity of the information. Leveraging bioaccess®'s expertise in Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies can significantly refine this strategy.
- Information Storage: Implement secure and compliant information storage solutions to safeguard participant details. Opt for cloud-based systems that offer encryption and stringent access controls. Bioaccess® emphasizes the critical importance of information protection, ensuring that diverse perspectives are considered in the formulation of these protocols, as highlighted by the expert panel meeting with 12 members.
- Data Cleaning: Establish rigorous processes for data cleaning to eliminate inaccuracies and outliers, which is vital for maintaining the integrity of your analysis. This step is crucial for promoting critical evaluation and enhancing the research's credibility, in line with bioaccess®'s commitment to comprehensive clinical investigation management.
- Statistical Analysis Plan: Develop a comprehensive statistical analysis plan that outlines the analytical approaches in accordance with your study objectives, incorporating both descriptive and inferential statistics. This plan should also consider the inclusion of quality of life metrics, as illustrated in the case analysis titled 'Quality of Life Assessments in Clinical Trials,' which highlights the comprehensive advantages of treatments beyond mere clinical results.
- Continuous Monitoring: Utilize real-time data observation tools to supervise participant adherence and device functionality throughout the study, allowing for timely interventions when necessary. This approach aligns with the need for evidence-based project selection, as cautioned by John Brownstein against distractions from shiny innovations. Bioaccess®'s experience in managing pivotal studies can provide invaluable insights into effective monitoring strategies.
- Reporting Outcomes: Prepare to present your findings transparently, adhering to established clinical study reporting guidelines. Ensure that both positive and negative results are included to provide a comprehensive view of the outcomes. As Sara Vaezy noted, it is essential to leverage our learnings to further the health system’s mission to serve all patients effectively. Bioaccess® also underscores the importance of adherence and transparency in addressing client issues, including grievance and information protection procedures, thereby strengthening trust in the reporting process, especially in the context of designing trials for wearables in Peru. By implementing these data management and analysis protocols, you can significantly enhance the reliability of your trial results and contribute valuable insights to the evolving field of wearable technology in healthcare.
Conclusion
Successfully navigating the regulatory landscape for wearable technology trials in Peru necessitates a comprehensive understanding of the guidelines from the Peruvian National Institute of Health. Key regulations, such as the Clinical Studies Regulation and Law No. 29459, are essential for ensuring compliance, participant safety, and ethical considerations, including informed consent and data protection.
Establishing clear objectives and designing appropriate parameters are critical for conducting effective trials. Engaging stakeholders and conducting pilot studies not only help refine approaches but also enhance the relevance of results. A combination of quantitative and qualitative methods improves patient engagement and ensures adherence to ethical standards, thereby increasing the credibility of outcomes.
Moreover, implementing robust data management and analysis protocols is vital for maintaining the integrity of findings. This involves a solid data collection strategy, secure storage, and comprehensive statistical analysis. Continuous monitoring and transparent reporting of results, whether positive or negative, are also crucial for advancing wearable technology in healthcare.
In conclusion, a structured approach emphasizing regulatory compliance, clear objectives, and effective data management is imperative for successful wearable technology trials in Peru. By focusing on these elements, researchers can adeptly navigate the complexities of clinical trials, drive innovation, and build trust within the healthcare landscape, ultimately enhancing trial outcomes and fostering responsible advancements in health tech.
Frequently Asked Questions
What is the primary organization overseeing clinical research regulations in Peru?
The primary organization is the Peruvian National Institute of Health (INS), which oversees clinical study approvals and compliance.
What is the Clinical Studies Regulation (CTR)?
The Clinical Studies Regulation (CTR) outlines the requirements for conducting clinical studies in Peru, emphasizing ethical considerations and participant safety.
What does Law No. 29459 regulate?
Law No. 29459 regulates the registration and supervision of medical devices, which is particularly relevant for portable technology.
Why is informed consent important in clinical trials?
Informed consent is crucial because all participants must fully understand the study's purpose, risks, and benefits before participating.
What should researchers know about information protection regulations?
Researchers should be familiar with information protection regulations to secure participant details, especially when using wearable devices that collect sensitive health information.
How does bioaccess® ensure information security?
bioaccess® is committed to maintaining information security and client confidence, addressing any issues through established grievance and data protection protocols.
What role does the INS's DIIS play in clinical studies?
The INS's DIIS supervises the ethical review process for clinical studies, emphasizing the importance of ethical adherence in study design.
Why is it important to understand the fee structures related to Institutional Ethics Committee evaluations?
Understanding the fee structures can provide valuable insights into potential expenses and fluctuations in the ethics review process, which is crucial for designing trials for wearables in Peru.
Where can researchers find comprehensive information on the regulatory environment in Peru?
Researchers can refer to resources such as the ClinRegs database and the official INS website for comprehensive information on the regulatory environment.
How can bioaccess® assist in the clinical study process?
bioaccess® specializes in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Post-Market Clinical Follow-Up Studies (PMCF), helping to streamline clinical study processes and ensure a successful transition from pilot study to commercialization.




