Overview
Effective patient recruitment for device trials is vital in ensuring diverse and representative participant pools. This diversity enhances the quality of data collected and significantly influences regulatory approvals. The article outlines various strategies such as:
- Community engagement
- Targeted advertising
- Clear communication
to address recruitment challenges. It emphasizes that tailored approaches and the integration of technology can dramatically improve enrollment success. By adopting these methods, clinical researchers can not only increase participant numbers but also ensure that the trials reflect a broader demographic, ultimately leading to more reliable outcomes.
Introduction
In the realm of clinical trials, particularly within the medical device sector, patient recruitment stands as a pivotal factor determining the success or failure of a study. As the healthcare landscape evolves, the necessity for effective recruitment strategies has never been more critical. Diverse participant pools are essential for generating valid data and achieving regulatory approvals, compelling stakeholders to navigate the complexities of engagement and communication.
Addressing common misconceptions and leveraging technological advancements, the path to successful recruitment is undeniably multifaceted. This article delves into the crucial aspects of patient recruitment, highlighting proven strategies, ethical considerations, and the significance of building trust, all aimed at enhancing participation and ultimately advancing medical innovation.
The Crucial Role of Patient Recruitment in Device Trials
In research studies, particularly within the medical device industry, patient recruitment for device trials stands as a fundamental element of success. Efficient hiring methods are crucial not only for ensuring that studies are sufficiently powered but also for enhancing the diversity and representativeness of the participant pool. This diversity is essential for generating accurate and dependable data that can significantly influence regulatory approvals and market success.
Current statistics reveal that the median number of changes in randomized studies is one, with a range from zero to eight, underscoring the necessity for flexibility in participant strategies. Moreover, a concerning 32% of patients reported that their doctors had shared information about clinical trials with them, despite 73% expressing a desire to learn about such opportunities from their healthcare providers. This gap highlights the significance of proactive communication in recruitment efforts.
A retrospective analysis of 393 randomized controlled trials (RCTs) closed to further enrollment identified key features associated with successful recruitment. Notably, government funding and participant compensation were positively correlated with enrollment success, while cancer research and unconventional methods presented challenges. The study employed the CatBoost regressor, which emerged as the best-performing model for predicting accrual percentages, emphasizing the need for flexible recruitment infrastructures and appropriate compensation strategies.
Furthermore, a study indicated that home visits were particularly appealing to Hispanic and non-white individuals, compared to their non-Hispanic and white counterparts (62.4% vs. 40.2%, p<.01 and 58.5% vs. 38.4%, p<.01 respectively). This insight underscores the necessity for customized recruitment approaches that resonate with various demographic groups.
The impact of patient recruitment for device trials on research outcomes cannot be overstated. Successful hiring not only accelerates patient recruitment for device trials but also enhances the overall quality of the data collected. As bioaccess® leverages its 20+ years of expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), it plays a crucial role in optimizing these strategies.
Moreover, the partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a prominent location for medical studies in Latin America, supported by Colombia's Minister of Health, further emphasizes the significance of efficient sourcing in promoting local economic development and healthcare enhancement. As the medical technology landscape continues to evolve, effective patient recruitment for device trials will be paramount for stakeholders aiming to navigate the complexities of research successfully.
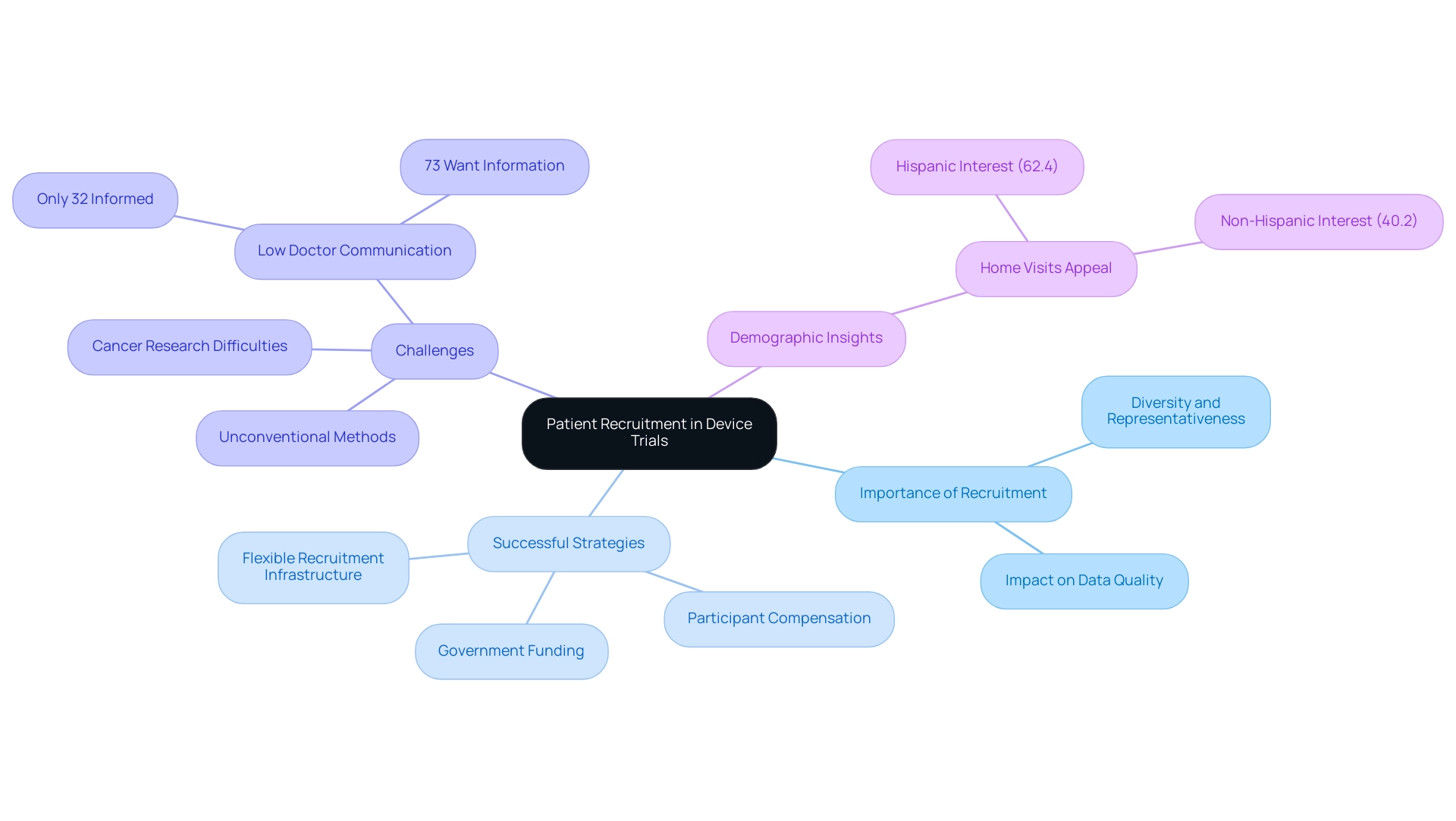
Identifying Challenges in Patient Recruitment for Clinical Trials
The challenges of patient recruitment for device trials significantly influence the success of medical studies. A notable concern is the lack of understanding among prospective participants; statistics reveal that a substantial portion of individuals remain unaware of ongoing research studies. This knowledge gap is further compounded by stringent eligibility criteria, which can limit participation even more.
Logistical barriers, such as difficulties with transportation and time commitments, play a critical role in deterring potential participants. Misunderstandings related to medical studies often exacerbate these challenges. Many individuals harbor fears about the unknown aspects of participation or may not fully comprehend the potential benefits, such as access to cutting-edge treatments and the opportunity to contribute to medical advancements. To effectively address these barriers, enhancing patient recruitment for device trials is essential.
This effort encompasses educational initiatives that clarify the clinical trial process, community engagement strategies that build trust and understanding, and customized approaches that resonate with diverse populations. For instance, a recent case study highlighted the success of a referral strategy where current members were encouraged to invite friends and family to join the study. This method, integrated into the final study visit procedures, achieved a remarkable 100% completion rate among those referred, underscoring the importance of participant satisfaction and engagement in patient recruitment for device trials.
Furthermore, the partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a prominent hub for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances the visibility of research studies but also contributes to local economic development through job creation and improved healthcare services. The comprehensive clinical trial management services provided by bioaccess™, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, play a crucial role in this endeavor.
Statistics indicate that in the worst hiring group (≥90% vs ≤10%), 22% had no resources utilized compared to 43% in the best group, illustrating the critical role of resource allocation in hiring success. Future studies could greatly benefit from systematic evaluations of engagement strategies, employing experimental designs and mixed-methods approaches to refine and enhance participation rates. As noted by Karen Soter, 'The authors thank the Clinical Outcomes Research and Education at Roseman University of Health Sciences College of Dental Medicine and Karen Soter for supporting this study.'
By adjusting efficient hiring approaches to different environments, patient recruitment for device trials can overcome typical obstacles and enhance enrollment results, ultimately advancing the field of medical technology.
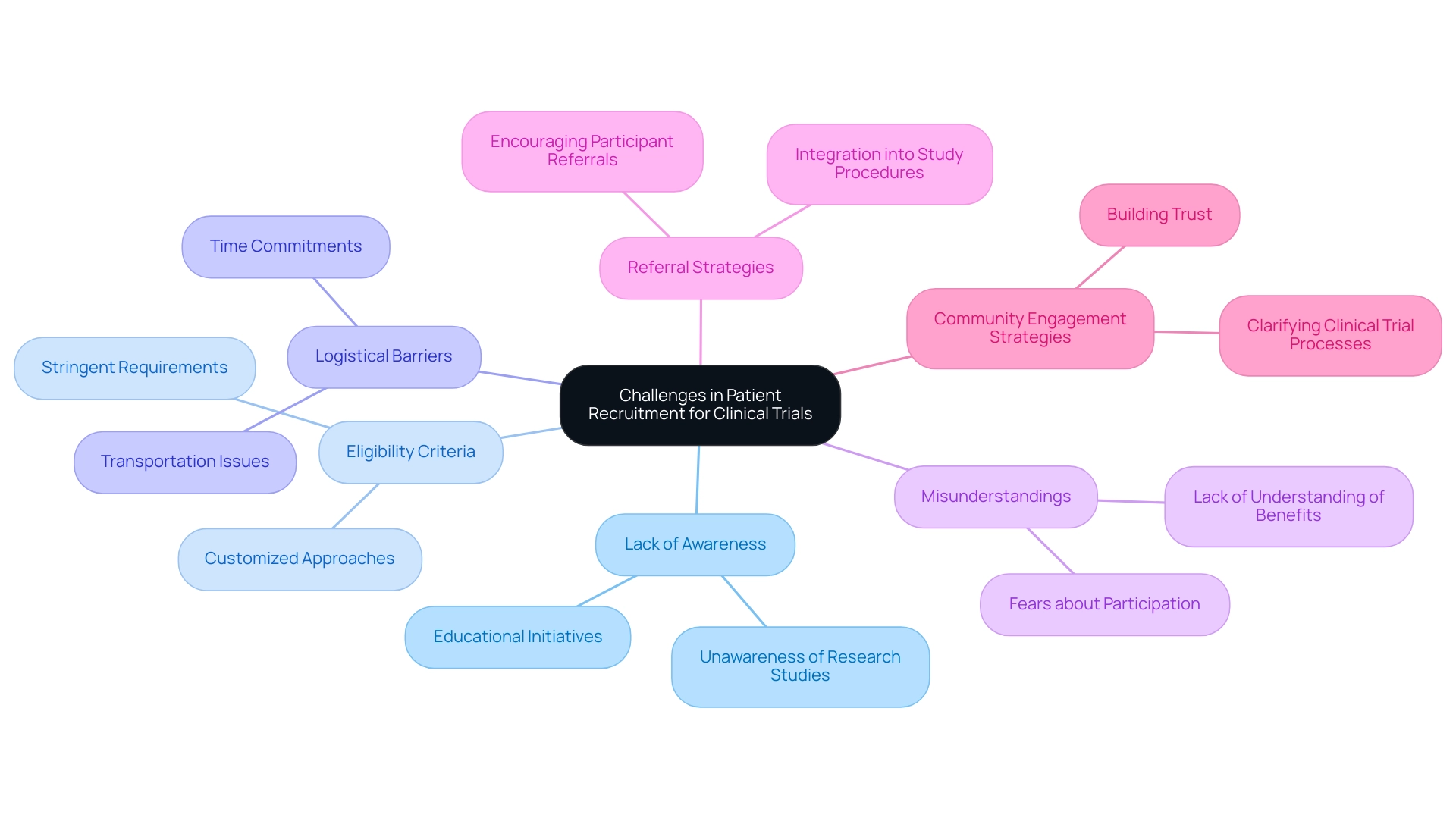
Proven Strategies for Effective Patient Recruitment
To enhance patient recruitment in clinical trials, several proven strategies can be effectively employed:
- Community Engagement: Collaborating with local healthcare providers and community organizations is crucial for raising awareness and building trust within the community. Interacting with these organizations not only nurtures connections but also enhances access to hard-to-reach groups, as demonstrated by research indicating that more than two-thirds (68.8%) of research studies were carried out in community environments. Additionally, five studies have effectively encompassed hard-to-reach populations, underscoring the importance of community engagement strategies. The collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a leading destination for clinical trials in Latin America exemplifies this approach, particularly in terms of patient recruitment for device trials, with support from Colombia's Minister of Health.
- Targeted Advertising: Utilizing digital platforms and social media to reach specific demographics can significantly enhance visibility among potential attendees. Targeted advertising has been shown to resonate particularly well with diverse populations, enhancing enrollment rates. For instance, home visits were notably more appealing to Hispanic and non-white individuals, highlighting the importance of tailored outreach strategies. As noted by Antidote, "When analyzing results by race, home visits appealed more to Hispanic and non-white individuals, compared with non-Hispanic and white survey respondents."
- Patient-Centric Communication: Clear, concise, and transparent communication regarding the study's purpose, procedures, and benefits is essential. This approach alleviates concerns and encourages involvement, as potential contributors are more likely to engage when they fully understand what is involved.
- Incentives: Providing compensation for time and travel can serve as a strong motivator for participation, particularly in studies that require multiple visits. This strategy recognizes the dedication of participants and can significantly improve hiring efforts.
- Streamlined Processes: Simplifying the enrollment process and providing adequate support can effectively reduce barriers to participation. Research indicates that 55% of investigators have over 10 years of experience, suggesting that leveraging their expertise in optimizing hiring processes can lead to more efficient trials. Notably, partnerships such as that of GlobalCare Clinical Trials with bioaccess™ have achieved over a 50% reduction in enrollment time and 95% retention rates, showcasing the impact of effective collaboration.
Alongside these strategies, continuous educational programs are essential for sustaining effective hiring, especially in rural areas where community staff turnover can present difficulties. The case study titled 'Challenges in Community Engagement' highlights these issues, emphasizing the need for continuous engagement and research into best practices to address the limitations faced in rural research environments. By tackling these challenges, the effectiveness of community engagement strategies in clinical trial participation can be further enhanced.
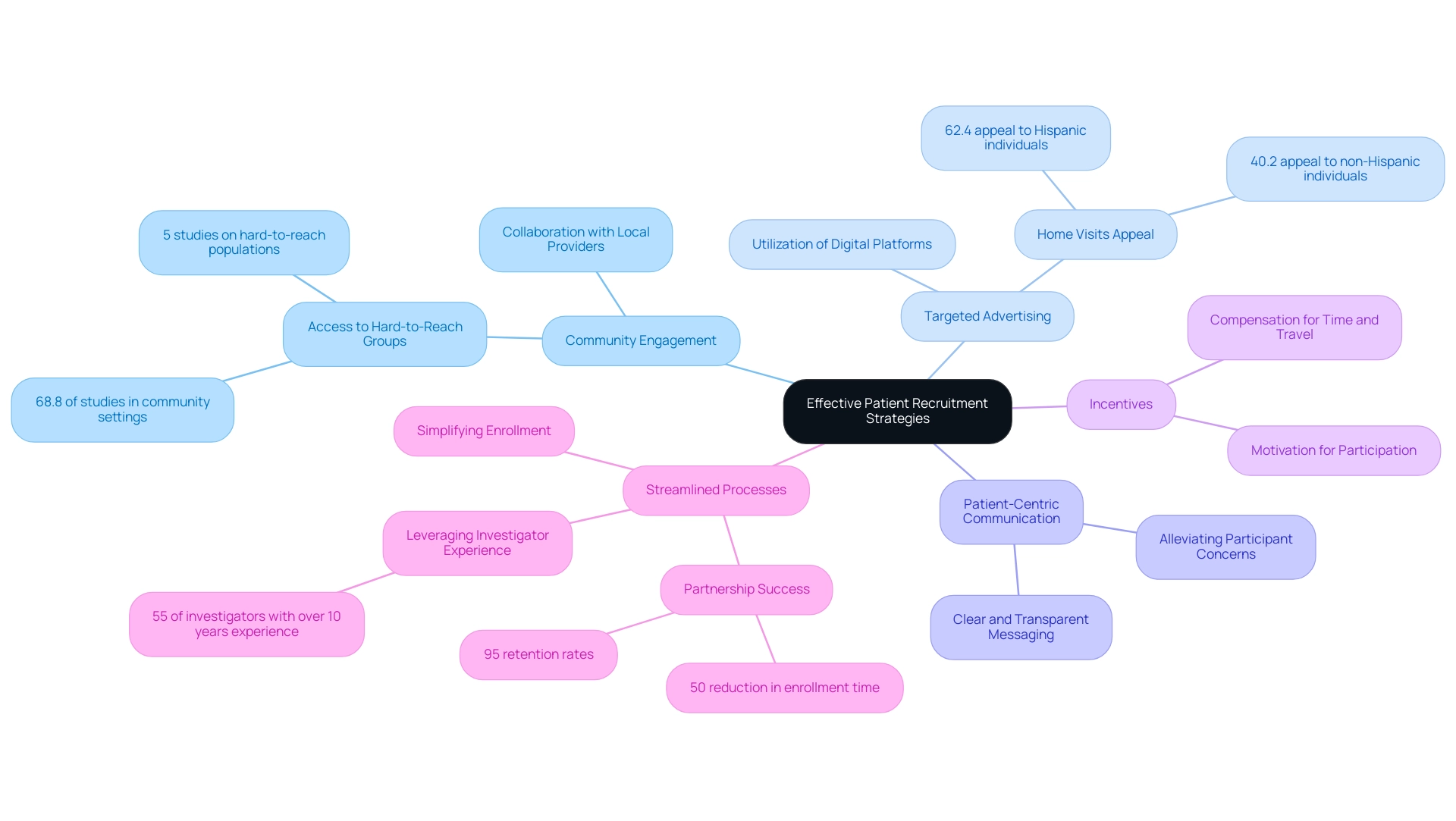
Leveraging Technology to Enhance Recruitment Efforts
Incorporating technology into patient engagement strategies dramatically enhances both efficiency and outreach. Electronic health records (EHRs) play a crucial role in swiftly identifying suitable candidates and optimizing the enrollment process. Recent statistics reveal that the median time from patient admission to sending a text message to eligible candidates was just 62 minutes, showcasing the potential for rapid engagement.
Social media platforms further bolster hiring initiatives by facilitating targeted outreach, allowing researchers to connect with potential contributors in their preferred online environments. Additionally, mobile applications enhance communication by providing reminders for study visits, significantly improving retention rates. The utilization of virtual messaging, such as email and SMS, has been demonstrated to boost enrollment rates in research studies, thereby enhancing patient recruitment for device trials and establishing it as a valuable resource in hiring strategies.
The rise of virtual assessments and telehealth alternatives has broadened access to research studies, particularly aiding patients in remote regions. A significant case study on stroke survivor enrollment illustrated that digital methods surpassed traditional approaches, leading to faster participant inclusion for a mobility study. This trend underscores the efficacy of technology in contemporary medical studies, making participation more attainable and appealing for diverse patient groups.
As we approach 2025, the integration of AI and digital resources, coupled with patient advocacy efforts, is transforming the landscape of medical studies, rendering them more patient-friendly and efficient. Florence Mowlem, PhD, Vice President of Science for ObvioHealth, emphasizes this shift, stating, "I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals."
Moreover, bioaccess® leverages its over 20 years of experience in overseeing extensive research services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies, to enhance participant engagement strategies. The partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a premier location for research studies in Latin America, supported by Colombia's Minister of Health. This initiative not only streamlines the hiring process but also facilitates patient recruitment for device trials, ensuring that innovative medical devices reach the market more swiftly and effectively.
Additionally, bioaccess's services align with INVIMA's regulatory roles, ensuring adherence and supervision in the research process, which is essential for the successful introduction of medical devices in Colombia. Statistics indicate that bioaccess has achieved over a 50% reduction in hiring time and 95% retention rates, demonstrating the effectiveness of their hiring strategies.
Building Trust and Transparency with Potential Participants
Building confidence with potential contributors is essential for successful patient recruitment in device trials within clinical studies. This trust can be cultivated through transparent communication regarding objectives, associated risks, and anticipated benefits. Clearly articulating how patient data will be utilized and ensuring confidentiality are vital steps in alleviating participant concerns.
Engaging with patient advocacy groups and community leaders not only enhances credibility but also fosters a sense of trust within the community.
Regular updates and maintaining open lines of communication throughout the process are crucial for strengthening trust and promoting ongoing participation. A study revealed that 73% of patients prefer to learn about research opportunities from their healthcare providers, underscoring the need for improved communication strategies in patient recruitment for device trials. Furthermore, as highlighted by industry specialists, openness in research studies significantly influences patient recruitment for device trials, with many individuals expressing a desire for clear information regarding their involvement.
As Elisa Cascade, Chief Product Officer at Advarra, noted, one of the greatest drivers of interest in studies was a patient’s dissatisfaction with their medication, particularly concerning bothersome side effects.
Incorporating these trust-building strategies not only enhances engagement but also contributes to the overall success of studies, ultimately improving patient recruitment for device trials in the market. Additionally, with 78.7% of participants viewing health-related videos on YouTube, leveraging modern communication strategies can further enhance trust and inform potential participants about research opportunities. The case study titled 'Patient Preferences for Study Information' emphasizes that patients often prefer to learn about research studies through their healthcare providers rather than advertisements or online communities, indicating that effective patient recruitment for device trials necessitates improved communication and outreach strategies in study enrollment.
Referencing the thorough supervision of research studies, including data analysis endorsement by the University of Kentucky Medical Internal Review Board, can also bolster the credibility of the recruitment process. Moreover, bioaccess® is committed to ensuring information security and client trust through robust data protection measures, addressing any concerns through our grievance procedures, and maintaining compliance with applicable laws. This commitment is essential in nurturing a reliable atmosphere for individuals, particularly in the context of research studies in Latin America and Colombia, where patient recruitment for device trials is increasingly emphasized by media coverage from Clinical Leader, highlighting the significance of openness and ethical standards in research.
Additionally, bioaccess® provides extensive study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project administration, and reporting, which are crucial for enhancing study design and execution. Understanding the role of INVIMA, the Colombia National Food and Drug Surveillance Institute, is also vital, as it oversees medical device regulation and classification, ensuring compliance with local health authority standards.
Ethical Considerations in Patient Recruitment
Ethical factors in patient recruitment for device trials are paramount for safeguarding the rights and well-being of individuals participating in clinical studies. A fundamental aspect of this process is obtaining informed consent, which necessitates that individuals fully comprehend the study's nature, objectives, and potential risks. Despite a generally positive perception of the informed consent process, studies reveal a concerning gap between patients' self-reported understanding and their actual knowledge; while 51.4% of patients rated their knowledge about the study as high, only 14.3% demonstrated a true understanding of the information provided.
Strategies for patient recruitment in device trials must be meticulously designed to avoid coercion, ensuring that individuals are not unduly influenced to join a trial. This is particularly critical in the Medtech sector, where the stakes can be exceptionally high. For instance, a review examining the impact of educational background on comprehension found that individuals with higher educational attainment typically exhibited a better understanding of informed consent components. This underscores the necessity of customizing communication strategies to enhance patient recruitment for device trials, catering to varying levels of education among prospective individuals.
Moreover, maintaining confidentiality and adhering to data protection regulations are essential elements in the patient recruitment process for device trials. As noted by Research staff 018, "Info leaflets are getting more complicated with GDPR/data protection information. It is almost impossible to make it shorter without risking rejection by ethics committee." This highlights the challenges faced in simplifying informed consent documents while ensuring compliance. At bioaccess®, we are committed to addressing any queries or concerns regarding the processing of individual information through our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. This commitment ensures that client concerns are handled with compliance and transparency.
These practices not only adhere to regulatory requirements but also enhance the credibility of the research, which is vital for patient recruitment in device trials and fosters trust among potential subjects. As the landscape of medical studies evolves, continuous research is recommended to empirically evaluate the differences between patients' true grasp of consent, their assurance in that grasp, and physicians' perceptions of their patients' understanding. By emphasizing ethical factors in hiring and implementing robust data security practices, medical studies can significantly improve patient recruitment for device trials, ultimately contributing to the success of medical device research.
Continuous Improvement: Adapting Recruitment Strategies for Success
Ongoing enhancement of patient recruitment strategies for device trials is essential for the success of clinical trials, particularly in the medical device sector. This process necessitates a systematic assessment of current hiring techniques, incorporating input from individuals and stakeholders to refine approaches. By utilizing data analytics to track hiring metrics, organizations gain valuable insights into the effectiveness of various strategies, enabling teams to discern what resonates with potential participants and what does not.
For instance, a recent partnership between bioaccess™ and Caribbean Health Group (CHG) has positioned Barranquilla as a leading destination for clinical studies in Latin America, demonstrating the effectiveness of comprehensive clinical study management services, including feasibility assessments, site selection, and compliance reviews. This collaboration, supported by Colombia's Minister of Health, aims to improve the selection process and overall success of studies in the region. A recent study within this initiative revealed that in-person hiring strategies achieved an impressive 100% completion rate, underscoring the effectiveness of direct engagement.
This case exemplifies how targeted hiring efforts can significantly enhance participant retention and overall trial success. Furthermore, the study met its enrollment target within twelve months, highlighting the importance of timely and efficient hiring processes. Additionally, a community service event prescreened 34 individuals, with 8 evaluated and 6 completing the study, resulting in a completion rate of 75%, illustrating the potential of community engagement in outreach efforts.
As we approach 2025, the emphasis on longitudinal data gathering and enhanced study information will be vital for improving patient enrollment. By consistently examining hiring effectiveness through data analysis, organizations can adjust their strategies to align more closely with individuals' needs and preferences. This proactive approach not only enhances hiring initiatives but also fosters a deeper understanding of the factors influencing participant involvement, ultimately aiding in patient recruitment for device trials in research studies.
The economic impact of these studies in Barranquilla is substantial, contributing to job creation and healthcare enhancement in the area. As noted by the Principal Investigator, "The clinical trial was approved by the Institutional Review Board of the university and registered on https://clinicaltrials.gov," emphasizing the critical role of regulatory compliance in recruitment strategies.
Conclusion
Patient recruitment is undeniably a critical factor in the success of clinical trials, particularly in the medical device sector. Effective recruitment strategies not only ensure adequate trial power but also promote diversity within participant pools, which is essential for generating valid and reliable data. The alarming statistics regarding patient awareness and the disparities between patient desires and actual recruitment efforts emphasize the urgent need for improved communication and engagement strategies.
The proven strategies discussed—community engagement, targeted advertising, and leveraging technology—showcase the multifaceted approach required to enhance recruitment efforts. By addressing logistical barriers and misconceptions, stakeholders can foster trust and transparency, ultimately leading to increased participation rates. Furthermore, the ethical considerations surrounding informed consent and participant privacy must remain at the forefront of recruitment strategies to ensure a respectful and compliant process.
As the healthcare landscape continues to evolve, the integration of data analytics and continuous improvement mechanisms will be pivotal in refining recruitment strategies. The collaboration between bioaccess® and Caribbean Health Group exemplifies how strategic partnerships can enhance recruitment processes, positioning regions like Barranquilla as key players in clinical trials.
In conclusion, the successful recruitment of participants in clinical trials transcends merely filling quotas; it is about building a sustainable framework that prioritizes patient engagement, ethical practices, and continuous improvement. By embracing these principles, stakeholders can navigate the complexities of clinical research more effectively, driving innovation and ultimately improving healthcare outcomes.
Frequently Asked Questions
Why is patient recruitment important for device trials in the medical device industry?
Patient recruitment is fundamental for the success of device trials as it ensures studies are sufficiently powered and enhances the diversity and representativeness of the participant pool, which is essential for generating accurate and reliable data that can influence regulatory approvals and market success.
What challenges are associated with patient recruitment for device trials?
Challenges include a lack of awareness among prospective participants about ongoing studies, stringent eligibility criteria, logistical barriers such as transportation difficulties, and misunderstandings regarding the clinical trial process.
How does diversity in participant recruitment impact research outcomes?
Diversity in participant recruitment is crucial for producing accurate and dependable data, which can significantly affect regulatory approvals and the overall success of the device in the market.
What are some effective strategies for improving patient recruitment?
Effective strategies include educational initiatives to clarify the clinical trial process, community engagement to build trust, customized approaches for diverse populations, and referral strategies that encourage current participants to invite others.
What role does compensation play in patient recruitment success?
Compensation has been positively correlated with enrollment success, as it can incentivize participation and help overcome some of the barriers that deter potential participants.
How can logistical barriers affect patient recruitment?
Logistical barriers, such as transportation issues and time commitments, can deter potential participants from joining studies, making it essential to address these challenges to improve recruitment rates.
What insights have been gained from studies regarding demographic preferences in recruitment?
Studies have shown that home visits are particularly appealing to Hispanic and non-white individuals, indicating the need for tailored recruitment approaches that resonate with various demographic groups.
What is the significance of the partnership between bioaccess™ and Caribbean Health Group?
This partnership aims to establish Barranquilla as a key location for medical studies in Latin America, enhancing the visibility of research studies and contributing to local economic development and healthcare improvements.
How can future studies enhance patient recruitment strategies?
Future studies could benefit from systematic evaluations of engagement strategies, employing experimental designs and mixed-methods approaches to refine participation rates and overcome typical obstacles in recruitment.
What is the impact of successful patient recruitment on research data quality?
Successful patient recruitment accelerates the recruitment process for device trials and enhances the overall quality of the data collected, leading to more reliable research outcomes.




