Overview
The article underscores the critical components and advantages of clinical trial design templates that researchers should leverage to elevate their studies. It asserts that these templates not only streamline the research process but also guarantee efficiency, consistency, compliance, and clarity. Such attributes are pivotal, ultimately culminating in enhanced outcomes in medical research and significant advancements in patient care.
Introduction
In the intricate world of medical research, the design of clinical trials serves as a crucial pillar determining the success of new interventions. As the landscape evolves and patient needs grow increasingly complex, grasping the fundamentals of clinical trial design becomes essential for researchers navigating this challenging terrain. This article explores:
- The core components underpinning effective trial designs
- The significance of utilizing templates for consistency and compliance
- Various methodologies that can enhance study outcomes
By examining these elements, researchers will be well-equipped to formulate robust protocols that not only meet regulatory standards but also drive meaningful advancements in healthcare.
Understanding Clinical Trial Design: A Foundation for Researchers
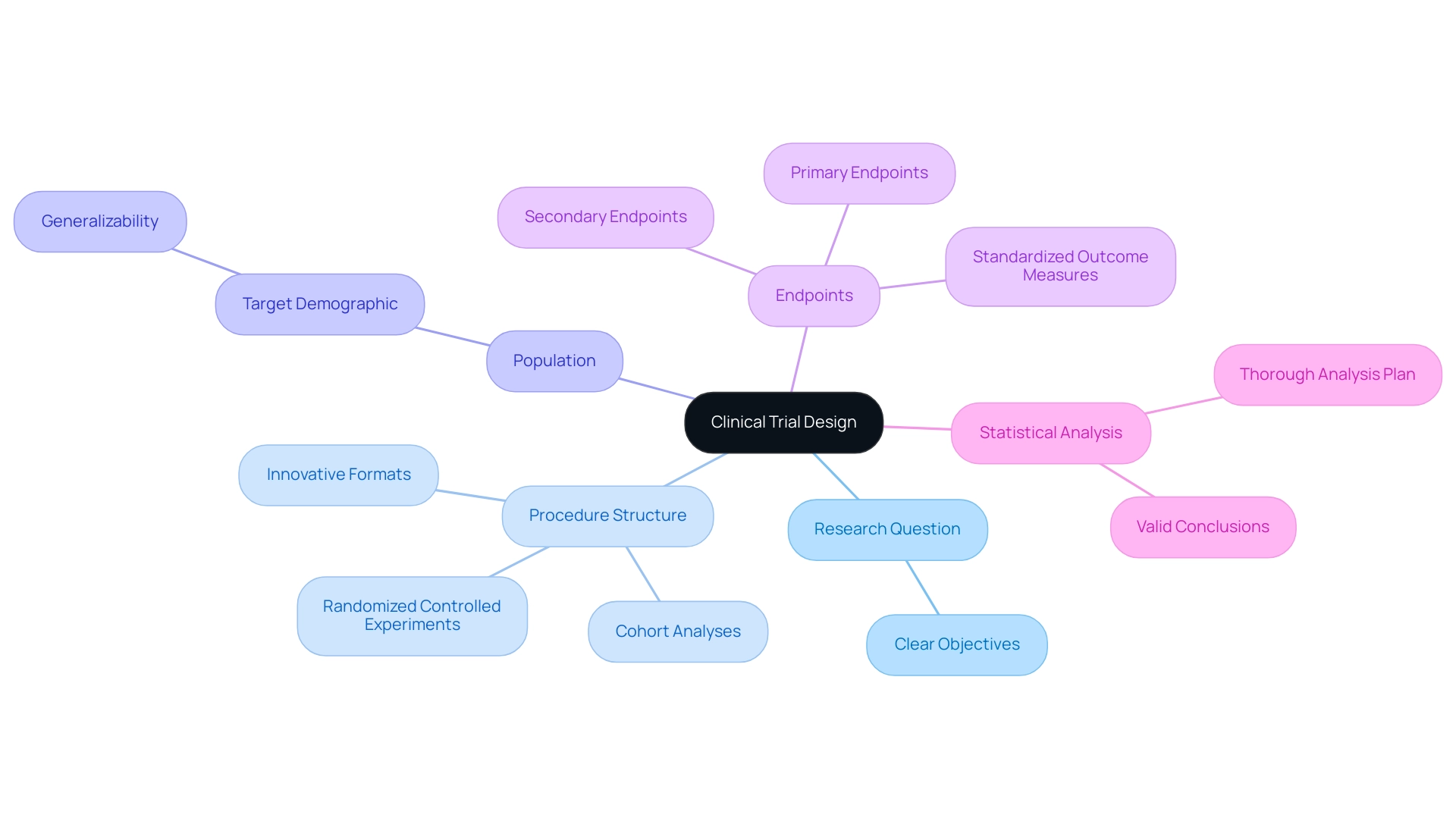
The clinical research framework acts as the foundation for investigations focused on evaluating the effectiveness and safety of medical interventions. It encompasses a structured framework that includes several critical components, each playing a pivotal role in the success of the experiment. In 2025, the emphasis on robust experimental procedure methodologies has never been more evident, particularly as the medical environment evolves and patient expectations rise.
Key Elements of Experimental Procedure:
- Research Question: Formulate a precise and clear research question that outlines the objectives of the investigation.
- Procedure Structure: Select the most suitable procedure structure, such as randomized controlled experiments or cohort analyses, to align with the research goals. Recent advancements have demonstrated that employing innovative formats can enhance data quality and relevance.
- Population: Define the target demographic carefully, ensuring that the selected population reflects the broader patient community to improve the generalizability of results.
- Endpoints: Establish both primary and secondary endpoints to measure the success of the intervention effectively. Standardizing outcome measures across studies is essential for creating medical guidelines and enabling future comparisons among interventions.
- Statistical Analysis: Formulate a thorough statistical analysis plan to guarantee that data is examined correctly, permitting valid conclusions to be drawn.
The significance of medical research methodology cannot be overstated. A well-organized framework not only enhances the reliability of the findings but also streamlines the regulatory approval process. For instance, bioaccess® specializes in managing various types of medical device research studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
With over 20 years of expertise in Medtech, bioaccess® offers specialized knowledge and adaptability to manage the intricacies of research protocols, particularly in Latin America, where regulatory organizations such as INVIMA play a crucial role in medical device supervision and categorization as a Level 4 health authority by PAHO/WHO.
Furthermore, grasping the subtleties of research protocols is crucial for investigators. It enables them to develop effective testing protocols and efficiently utilize clinical trial design templates. For instance, the selection between a parallel or crossover design can significantly affect the study's results and interpretations, underscoring the necessity for careful planning.
As the biotech sector grapples with essential business model challenges, enhancing study design through clinical trial design templates has become a vital strategy for achieving commercial success. By concentrating on effective methodologies, researchers can enhance the likelihood of successful experiments, ultimately benefiting the advancement of medical technologies and improving patient outcomes. Notably, 45% of Alcon's data is recorded on the same day as the visit date, emphasizing the importance of prompt data management in research studies.
Additionally, Joseph Tucker, CEO of Enveric, noted that the FDA's scrutiny of the psychedelic space reflects the regulatory challenges that researchers must navigate. By addressing these complexities, researchers can better position their investigations for success.
The Importance of Templates in Clinical Trial Design
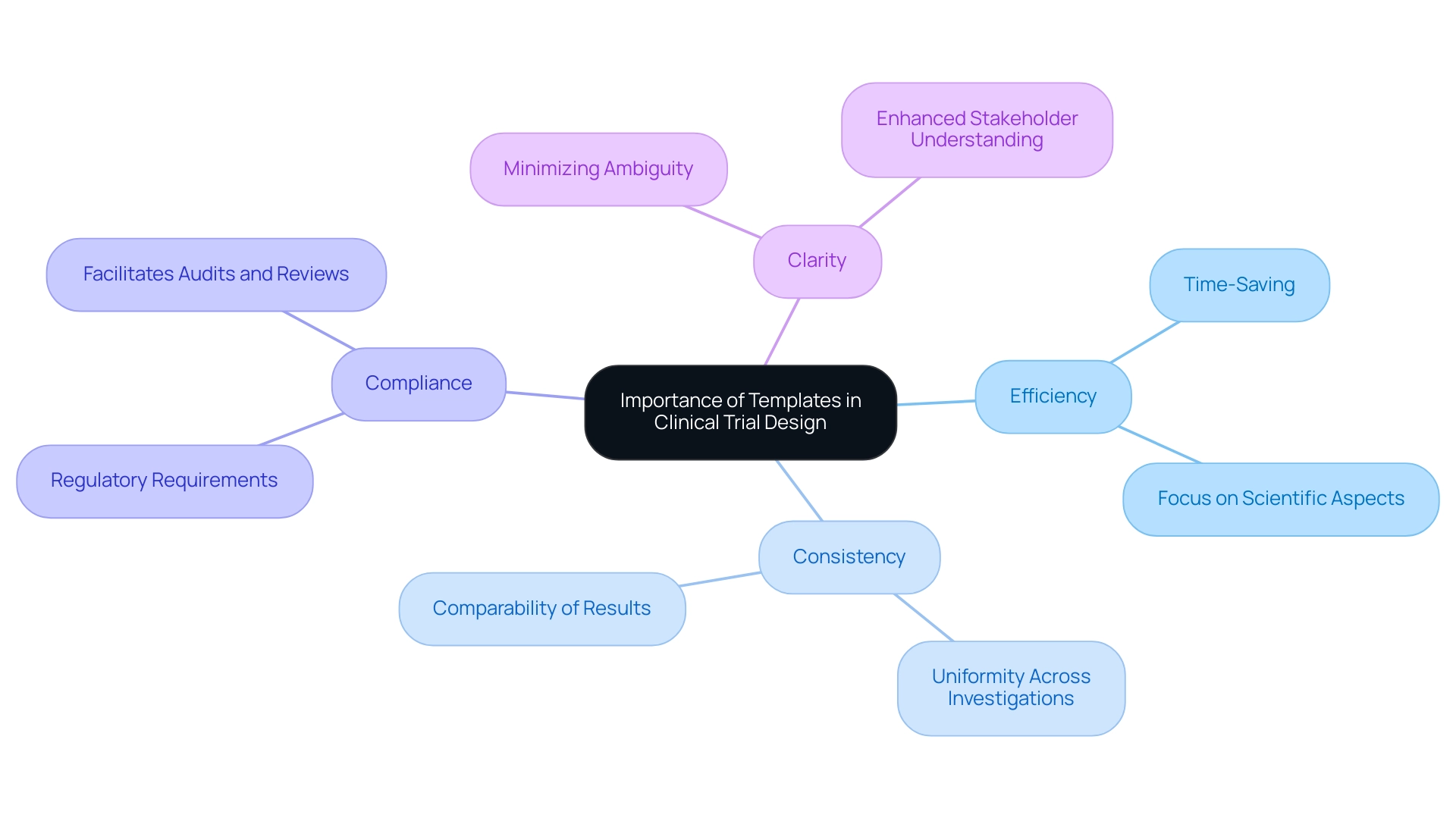
Clinical trial design templates are essential tools in study design, equipping researchers with pre-structured formats that streamline the creation of protocols, consent forms, and data collection sheets. By utilizing these templates, researchers can systematically incorporate all critical components, significantly minimizing the risk of oversight. Additionally, clinical trial design templates foster consistency across diverse research efforts, a crucial factor in maintaining regulatory compliance and ensuring data integrity.
In the realm of clinical research management, bioaccess offers a comprehensive suite of services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting. This holistic approach not only boosts the efficiency of clinical trial design templates but also guarantees that all regulatory requirements are fulfilled, facilitating smoother audits and reviews.
Benefits of Using Templates:
- Efficiency: Templates save valuable time by providing a ready-made structure, allowing researchers to concentrate on the scientific aspects of their studies rather than administrative tasks.
- Consistency: Utilizing templates ensures uniformity across various investigations, which is vital for comparing results and upholding the integrity of the research process.
- Compliance: Templates are crafted to meet regulatory requirements, ensuring that all necessary elements are included, thus enabling smoother audits and reviews.
- Clarity: By minimizing ambiguity in protocol development, templates enhance understanding among all stakeholders regarding the study's objectives and methodologies.
The integration of clinical trial design templates not only enhances operational efficiency but also aligns with the evolving landscape of medical research, where standardization and clarity are paramount. As Bree Burks, Vice President of Site Strategy at Veeva, notes, "As sponsors reconsider their site engagement strategies, they will emphasize consistent site technology and standardization across sponsors for all studies." This insight underscores the strategic application of templates, which will remain vital in improving research project planning and implementation.
Key Components of an Effective Clinical Trial Design Template
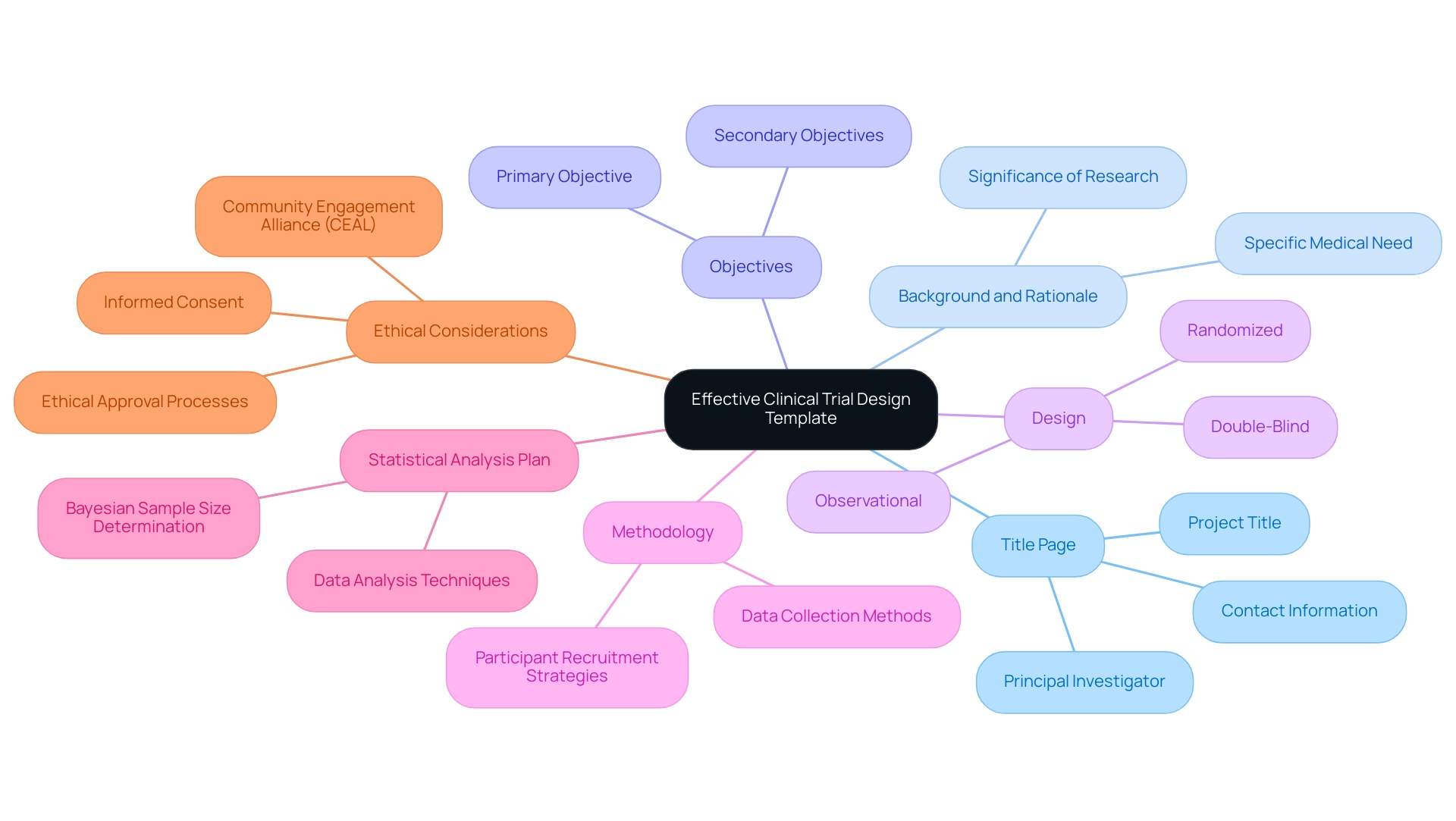
An effective clinical trial design template is crucial for ensuring thoroughness and compliance in clinical trial design. It should encompass several essential components that guide researchers through the planning and execution phases. The key elements to be included are:
- Title Page: This section should feature the project title, principal investigator, and relevant contact information, providing a clear point of reference for all stakeholders.
- Background and Rationale: A compelling justification for the research is necessary, outlining its significance and the specific medical need it addresses. This context helps to align the research with broader healthcare goals.
- Objectives: Clearly defined primary and secondary objectives are vital. These objectives guide the research focus and assist in measuring the project's success.
- Design: A detailed description of the chosen design—such as randomized, double-blind, or observational—ensures that the methodology aligns with the project's objectives and enhances the reliability of the results.
- Methodology: This comprehensive outline should detail procedures, including participant recruitment strategies and data collection methods. A well-structured methodology is essential for replicability and validity.
- Statistical Analysis Plan: This component specifies how data will be analyzed to answer the research questions. Incorporating Bayesian sample size determination techniques can provide alternatives to traditional power calculations, enhancing the robustness of the research design. These techniques allow for more flexible and informed decision-making regarding sample sizes, which can lead to more efficient studies.
- Ethical Considerations: Addressing informed consent and the ethical approval processes is critical. This ensures that the research adheres to ethical standards and fosters trust among participants. The Community Engagement Alliance (CEAL) initiative demonstrates how nurturing community partnerships can improve ethical involvement in medical studies, especially among marginalized groups. Moreover, with the FDA's Single IRB Requirement anticipated to be enforced in 2025, researchers must remain aware of compliance to ensure their projects meet regulatory standards.
By incorporating these essential components into clinical trial design templates, researchers can enhance their planning effectiveness, ultimately leading to more successful outcomes in the Medtech sector. With over 20 years of experience, bioaccess® understands the importance of these elements in advancing medical devices and improving patient outcomes. bioaccess® specializes in various investigations, including Early-Feasibility Evaluations, First-In-Human Trials, Pilot Assessments, Pivotal Research, and Post-Market Clinical Follow-Up Evaluations.
Their extensive management services for research include feasibility assessments, site selection, compliance evaluations, setup, import permits, project coordination, and reporting.
Exploring Different Types of Clinical Trial Designs
Clinical experiments can be classified into various formats, each offering unique benefits and applications that are essential for effective research planning. Understanding these clinical trial design templates empowers investigators to tailor their study frameworks to meet specific objectives and regulatory standards.
Common Research Study Layouts:
- Randomized Controlled Studies (RCSs): RCSs are recognized as the gold standard in medical research. Participants are randomly assigned to either treatment or control groups, minimizing bias and enhancing the reliability of results. As of 2025, RCTs continue to dominate clinical research methodologies, with approximately 60% of all experiments employing this method, underscoring its efficacy in establishing causal relationships between interventions and outcomes.
- Cohort Analyses: These observational studies track a group of participants over time to evaluate outcomes associated with specific exposures or interventions. They are particularly valuable for understanding long-term effects and are increasingly utilized alongside RCTs to provide supplementary data.
- Cross-Over Trials: In this design, participants receive multiple treatments sequentially, effectively serving as their own controls. This approach can enhance the effectiveness of experiments and is especially advantageous in research where individual variability is pronounced.
- Factorial Structures: This framework enables researchers to examine multiple interventions simultaneously, allowing for the assessment of interactions among treatments. Factorial designs are gaining traction as they can yield comprehensive insights while optimizing resource utilization.
- Adaptive Trials: These innovative designs allow for modifications based on interim results, enhancing flexibility and efficiency. With regulatory bodies increasingly endorsing adaptive approaches, their application is expected to rise, particularly in complex therapeutic areas.
Comprehensive Research Management Services
At bioaccess, we offer a complete suite of research management services that enhance these frameworks, including feasibility studies, site selection, compliance assessments, setup processes, import permits, project oversight, and reporting. Our expertise ensures that each study is meticulously organized and executed, addressing the unique challenges posed by various regulatory frameworks and improving the overall quality of medical research.
Expert Insights and Trends
Experts emphasize the importance of selecting the appropriate study format based on the research question and target population. Kevin Coker, an Innovation Strategist at MD Anderson Cancer Center, observes that while conducting studies across different countries can be advantageous, it presents challenges in navigating diverse regulations and cultural standards. This highlights the necessity for well-considered clinical trial design templates that address these complexities, especially in light of anticipated regulatory differences that complicate global studies.
Moreover, the FDA's draft guidance on Diversity Action Plans encourages sponsors to enroll more participants from underrepresented groups, underscoring the critical importance of diversity in research frameworks. This focus on inclusivity not only enhances the applicability of research findings but also addresses ethical considerations in medical investigations.
Case Examples and Results
A significant case example illustrates the proactive management of issues using historical data in medical evaluations. By defining thresholds and sharing data across departments, sponsors can effectively monitor trends and document remediation efforts. This approach not only enhances data quality by identifying issues early but also leads to quicker approvals and reduced timelines, demonstrating the essential role of planning in research success.
In summary, the selection of a research framework is crucial for achieving reliable and practical results. As the landscape of medical research evolves, staying abreast of the latest trends and best practices will enable researchers to enhance their project planning and execution, supported by comprehensive services from bioaccess.

Regulatory Considerations for Clinical Trial Design Templates
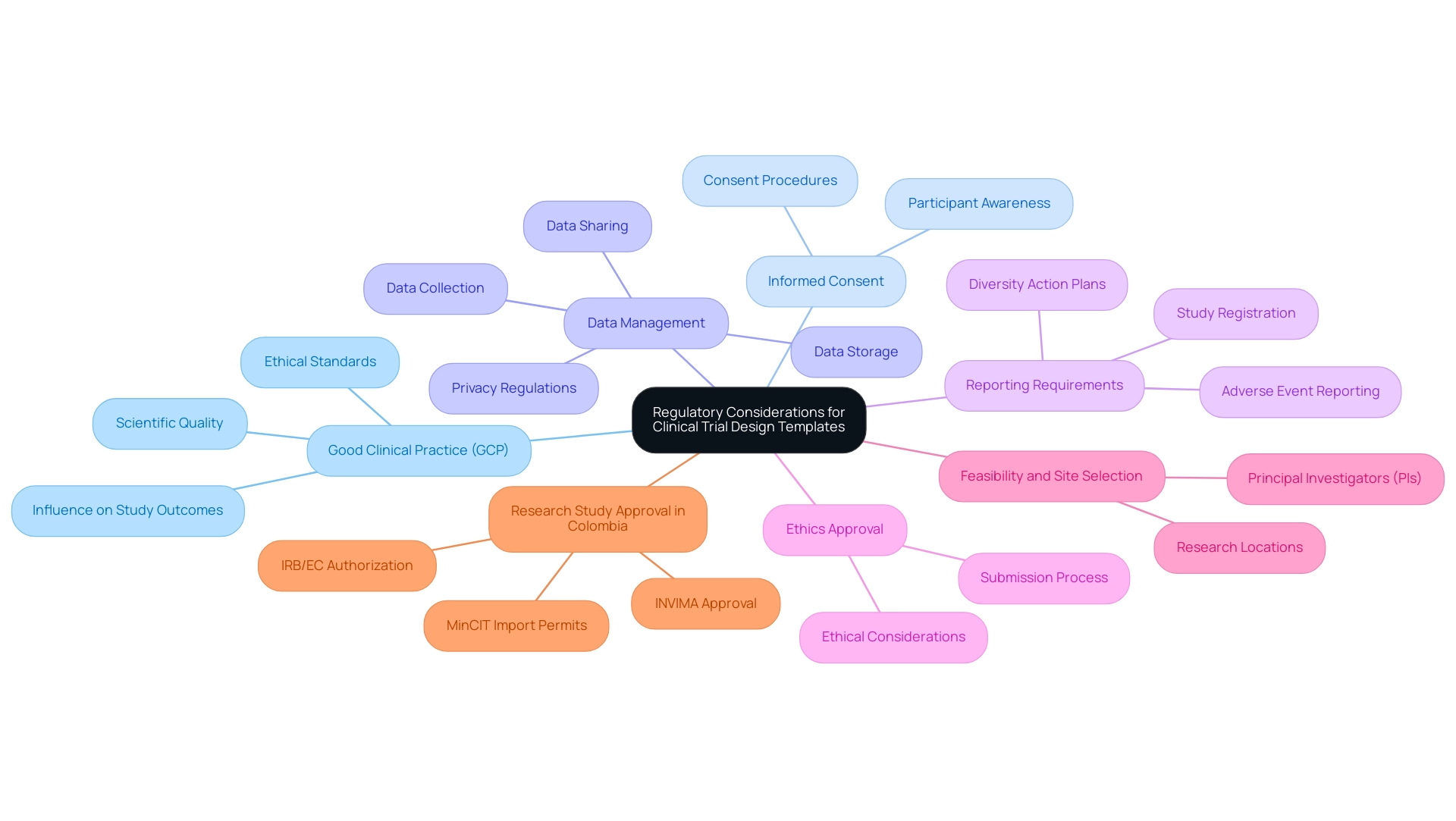
Regulatory compliance serves as a fundamental pillar in the creation of clinical trial design templates for medical studies, ensuring that researchers align these templates with the stringent guidelines established by regulatory authorities such as the FDA and EMA. This adherence not only safeguards participant welfare but also enhances the credibility and reliability of study outcomes.
Key Regulatory Considerations:
- Good Clinical Practice (GCP): Compliance with GCP is essential, as it encompasses ethical and scientific quality standards that govern the design, conduct, and reporting of clinical trials. GCP adherence significantly influences study outcomes, fostering trust in the data generated.
- Informed Consent: A robust informed consent process is vital. Clinical trial design templates must clearly outline procedures for obtaining and documenting participant consent, ensuring that participants are fully aware of the nature, risks, and benefits of the research.
- Data Management: Effective data management protocols are crucial. Templates should detail processes for data collection, storage, and sharing, all while adhering to privacy regulations to protect participant information. bioaccess® is dedicated to guaranteeing information security and client trust throughout the research process.
- Reporting Requirements: Comprehensive clinical trial design templates must include sections dedicated to adverse event reporting and study registration, facilitating transparency and accountability throughout the research. The FDA encourages sponsors to create Diversity Action Plans to enroll participants from a variety of backgrounds, which is essential for enhancing the diversity of clinical study populations.
- Ethics Approval: It is essential that clinical trial design templates simplify the submission process for ethics review and approval, ensuring that all ethical considerations are adequately addressed before the study commences. bioaccess® provides services that encompass set-up, start-up, and acquiring necessary approvals from ethics committees and health ministries, ensuring adherence to local regulations.
- Feasibility and Site Selection: bioaccess® offers expertise in the feasibility and selection of research locations and principal investigators (PIs), ensuring that studies are conducted in appropriate environments that meet regulatory standards.
- Research Study Approval in Colombia: Understanding the process for obtaining research study approval in Colombia, including IRB/EC authorization, INVIMA approval, and MinCIT import permits, is crucial for successful study execution.
A case study from the NIA emphasizes effective recruitment and retention strategies focused on enhancing participant diversity in medical studies, especially among underrepresented groups. These strategies improve the variety of research participant groups, ensuring that study results are relevant to a wider demographic and addressing health disparities.
As Samruddhi Yardi observes, "Grasping the regulatory environment is essential for researchers to maneuver through the intricacies of medical study structure efficiently."
By incorporating these regulatory factors into the research framework, investigators can improve the quality and integrity of their investigations, ultimately contributing to more dependable and relevant research outcomes. The expertise of professionals like Ana Criado and Katherine Ruiz further supports bioaccess®'s commitment to navigating these complexities effectively.
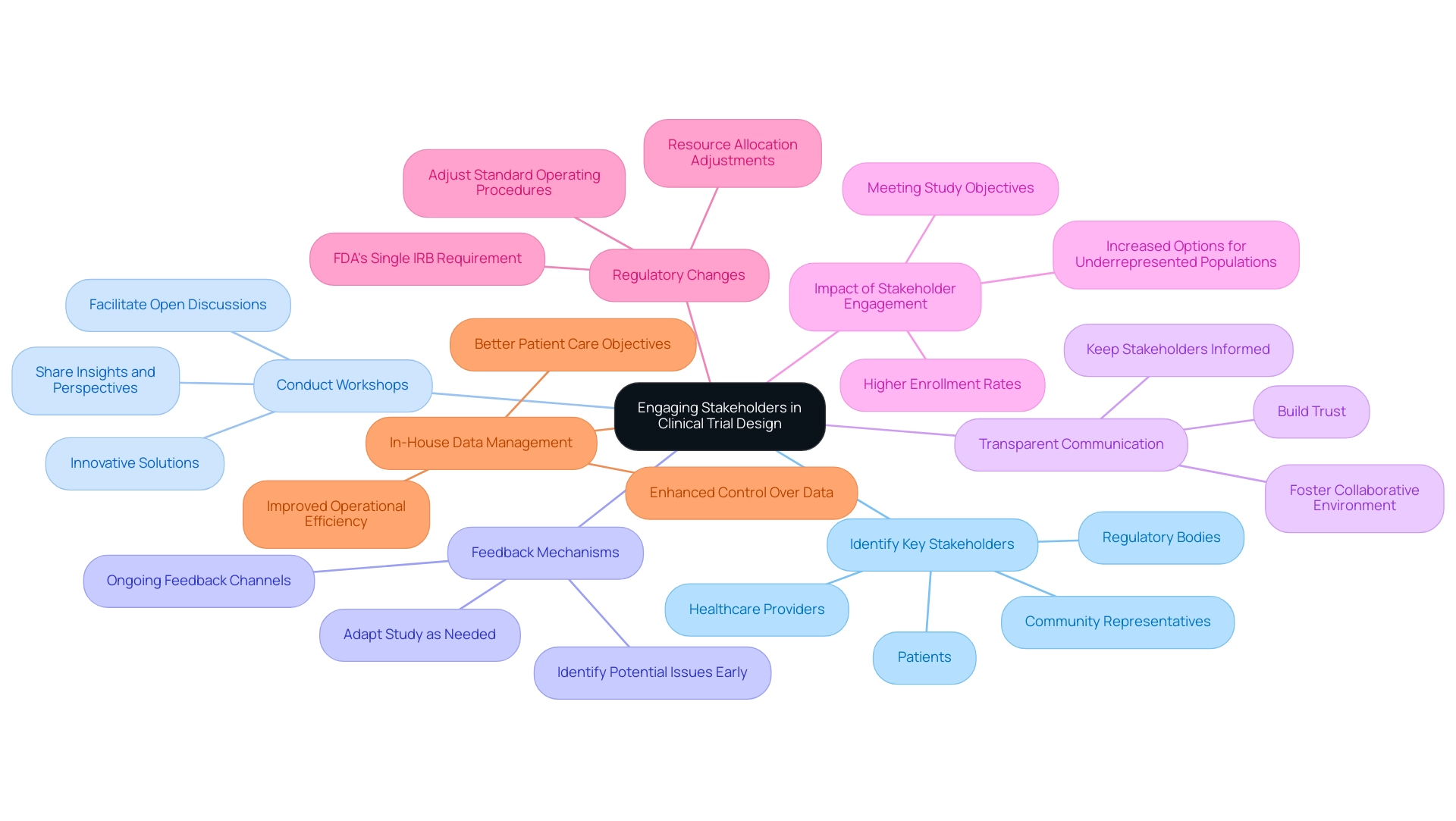
Engaging Stakeholders in Clinical Trial Design
Stakeholder involvement is a crucial component in clinical research, significantly influencing the overall success of the project. By involving key stakeholders from the outset, researchers can foster collaboration and ensure that the trial addresses pertinent questions that resonate with all parties involved.
Strategies for Engaging Stakeholders:
- Identify Key Stakeholders: Recognize and include a diverse group of stakeholders such as patients, healthcare providers, regulatory bodies, and community representatives. This diversity enriches the research framework and enhances its significance.
- Conduct Workshops: Organize workshops to facilitate open discussions, allowing stakeholders to share their insights and perspectives on study design and objectives. This collaborative approach can lead to innovative solutions and enhanced results.
- Feedback Mechanisms: Establish robust channels for ongoing feedback throughout the testing process. Regularly soliciting input from stakeholders can help identify potential issues early and adapt the study as needed, ultimately enhancing its effectiveness.
- Transparent Communication: Maintain open lines of communication with stakeholders by keeping them informed about the progress and findings of the study. This transparency builds trust and fosters a collaborative environment, which is essential for successful stakeholder engagement.
The effect of stakeholder participation on clinical study success rates cannot be overstated. Studies indicate that projects with active stakeholder engagement are more likely to meet their objectives and achieve higher enrollment rates. In 2025, underrepresented research populations will have increased options for onboarding and visits, further emphasizing the need for inclusive engagement strategies.
Furthermore, the significance of patient participation in research studies is progressively acknowledged. Engaging patients not only enhances the relevance of the research but also improves patient care outcomes. As Vivienne van der Walle, a prominent figure in medical research, notes, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care."
This highlights the critical role of patient feedback in shaping effective stakeholder engagement strategies.
In light of the FDA's upcoming Single IRB Requirement, stakeholders will need to adjust standard operating procedures and resource allocation to align with these new practices. This requirement necessitates a more coordinated approach to stakeholder engagement, ensuring that all parties are informed and involved in the decision-making process.
At bioaccess, our extensive research oversight services include feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting. These services not only simplify the research process but also improve stakeholder involvement by ensuring that all regulatory and logistical elements are carefully managed. A notable trend among sponsors is the movement towards in-house data management processes, which enhances control over data and improves operational efficiency.
By bringing research in-house, sponsors can ensure higher quality data management and better deliver on patient care objectives. This shift not only demonstrates the essential role of stakeholder involvement in research planning but also highlights the necessity for sponsors to modify their strategies to ensure effective communication and cooperation with all participants involved. Moreover, the influence of Medtech research on local economies, including job creation and healthcare enhancement, underscores the wider relevance of these evaluations, emphasizing the necessity of stakeholder involvement in attaining both research and community objectives.
Best Practices for Using Clinical Trial Design Templates
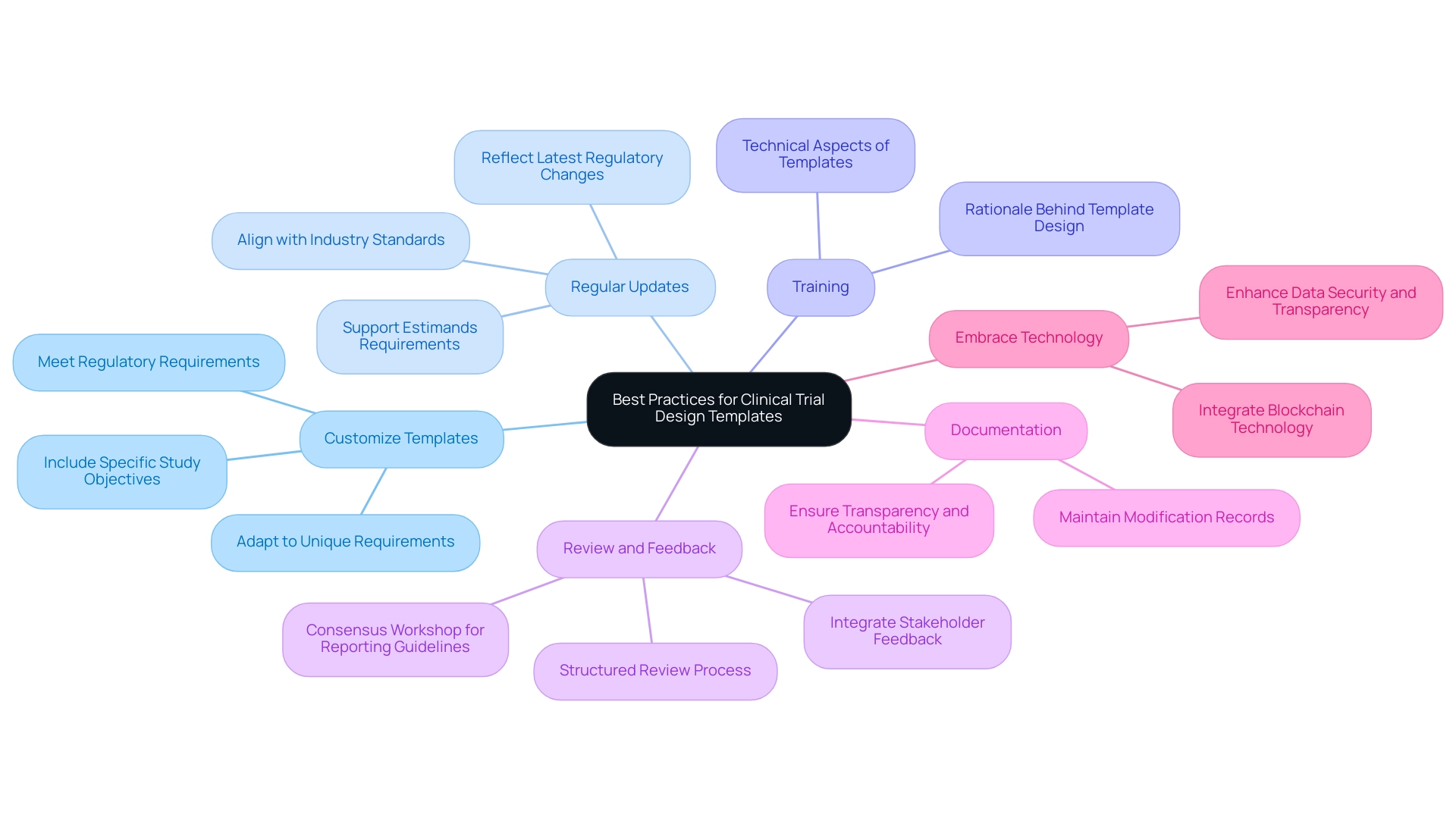
To maximize the effectiveness of clinical trial design templates, researchers must implement several best practices that align with the comprehensive clinical trial management services offered by bioaccess:
Best Practices:
- Customize Templates: Adapt templates to meet the unique requirements of each trial while preserving critical components. Customization is crucial, as it enables the inclusion of specific study objectives and regulatory requirements, ultimately improving the relevance and applicability of the research framework.
- Regular Updates: Ensure that clinical trial design templates are consistently updated to reflect the latest regulatory changes and industry standards, particularly in light of the evolving Medical Device Regulation (MDR). Staying current is essential for compliance and can significantly affect the success of clinical studies. The recent SAP updates emphasize better alignment of statistical analyses to support estimands requirements, highlighting the need for regular template revisions.
- Training: Offer comprehensive training for research teams on the effective use of clinical trial design templates. This training should cover not only the technical aspects but also the rationale behind template design, fostering a deeper understanding of their importance in the research process.
- Review and Feedback: Implement a structured process for reviewing clinical trial design templates and integrating feedback from all stakeholders. This collaborative approach can lead to enhanced template functionality and greater alignment with objectives. For instance, the Consensus Workshop for Finalizing Reporting Guidelines illustrates how expert collaboration can refine reporting standards and enhance template effectiveness.
- Documentation: Maintain meticulous documentation of any modifications made to the clinical trial design templates. This practice guarantees transparency and accountability, which are essential for regulatory compliance and for preserving the integrity of the process. This is particularly important in the context of bioaccess's services, which include compliance reviews and project management.
- Embrace Technology: Consider the integration of blockchain technology in research studies to enhance data security and transparency. As the industry evolves, utilizing such innovations can significantly enhance the integrity of testing processes.
By following these best practices, researchers can improve the quality and efficiency of their studies, ultimately resulting in more successful outcomes and quicker progress in medical technology. Bioaccess, as a leader in medtech clinical research in Latin America, emphasizes the importance of these practices in fostering innovation and regulatory excellence. As Bree Burks, Vice President of Site Strategy at Veeva, notes, "As sponsors rethink their site engagement strategies in 2025, they will prioritize consistent site technology and standardization across sponsors for all trials," underscoring the importance of these practices in the current landscape.
Conclusion
The exploration of clinical trial design underscores its foundational role in advancing medical research and improving patient outcomes. By delving into the core components of effective trial design, researchers can formulate precise research questions, select appropriate methodologies, and define target populations—elements essential for robust study outcomes. The utilization of templates emerges as a vital strategy, enhancing consistency, compliance, and clarity across various studies while streamlining the protocol development process.
Engaging stakeholders throughout the trial design is equally critical. Fostering collaboration with patients, healthcare providers, and regulatory bodies ensures that trials are relevant and address the needs of diverse populations. The incorporation of best practices in using clinical trial design templates further amplifies the effectiveness of studies, allowing for customization, regular updates, and thorough training for research teams.
As the landscape of clinical trials continues to evolve, staying informed about regulatory considerations and innovative methodologies empowers researchers to navigate complexities successfully. Ultimately, a well-designed clinical trial not only meets regulatory standards but also contributes significantly to the advancement of medical technologies and the enhancement of healthcare. The commitment to optimizing clinical trial design is imperative for driving meaningful improvements in patient care and achieving success in the highly competitive field of medical research.
Frequently Asked Questions
What is the purpose of the clinical research framework?
The clinical research framework serves as the foundation for investigations aimed at evaluating the effectiveness and safety of medical interventions, incorporating a structured approach that includes critical components essential for the experiment's success.
What are the key elements of experimental procedure in clinical research?
The key elements include: Research Question: A clear and precise question outlining the investigation's objectives. Procedure Structure: Choosing suitable structures like randomized controlled experiments or cohort analyses. Population: Defining a target demographic that reflects the broader patient community. Endpoints: Establishing primary and secondary endpoints for measuring intervention success. Statistical Analysis: Formulating a thorough plan for data examination to draw valid conclusions.
Why is medical research methodology important?
Medical research methodology enhances the reliability of findings and streamlines the regulatory approval process, making it crucial for successful investigations.
What services does bioaccess offer in clinical research?
Bioaccess provides a comprehensive suite of services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting.
How do clinical trial design templates benefit researchers?
The benefits of using clinical trial design templates include: Efficiency: Saving time by providing a ready-made structure. Consistency: Ensuring uniformity across various investigations. Compliance: Meeting regulatory requirements for smoother audits and reviews. Clarity: Minimizing ambiguity in protocol development.
What role does regulatory compliance play in clinical research?
Regulatory compliance is vital for maintaining data integrity and ensuring that all necessary elements are included in research protocols, facilitating smoother audits and reviews.
How does the selection of study design impact clinical research?
The choice between different study designs, such as parallel or crossover, can significantly affect the results and interpretations of the study, highlighting the need for careful planning.
What is the significance of timely data management in research studies?
Prompt data management, such as recording data on the same day as visits, is crucial for maintaining the integrity and relevance of research findings.
What challenges do researchers face in the regulatory landscape?
Researchers must navigate regulatory challenges, such as the scrutiny from organizations like the FDA, particularly in emerging areas like psychedelics, to position their investigations for success.

