Overview
The essential steps in trial design for successful clinical research are critical to ensuring robust outcomes. These include:
- Formulating a clear hypothesis
- Implementing randomization and blinding
- Establishing precise objectives and endpoints
Such elements are not merely procedural; they are foundational for minimizing bias and enhancing data integrity. Moreover, they ensure compliance with regulatory standards, which is paramount in today's Medtech landscape. Ultimately, these practices lead to reliable results that can significantly inform medical decisions and facilitate regulatory approvals.
Introduction
As the landscape of clinical research evolves, the design and execution of clinical trials have grown increasingly complex. This complexity demands a thorough understanding of core principles and regulatory requirements. Effective trial design relies heavily on foundational elements such as:
- Clear hypothesis formulation
- Randomization
- Blinding
These are essential components for producing valid and reliable results. With a growing emphasis on data integrity and ethical practices, navigating the intricacies of clinical trial protocols has never been more critical. This article delves into the essential components of clinical trial design, from establishing clear objectives and endpoints to conducting rigorous planning and risk assessments. Ultimately, it sheds light on how these practices contribute to successful outcomes in the rapidly advancing field of medical research.
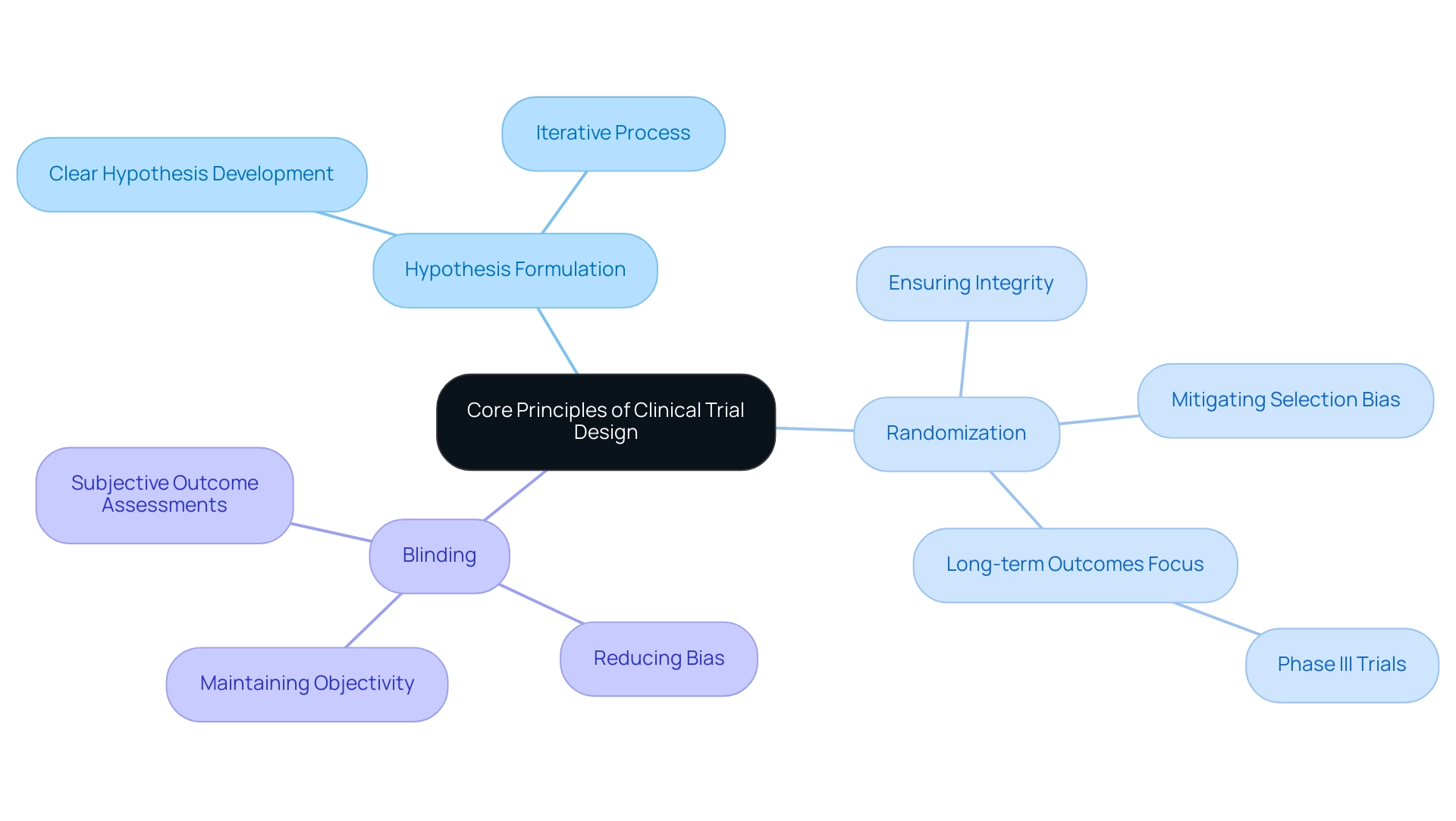
Understanding the Core Principles of Clinical Trial Design
Effective clinical study design incorporates essential steps in trial design, which encompass the formulation of a clear hypothesis, the critical role of randomization, and the necessity of blinding. These elements are crucial for generating valid and reliable results. Notably, randomization is vital as it mitigates selection bias, ensuring that participants are assigned to treatment groups without systematic differences. This process enhances the integrity of the study's findings, rendering them more robust against scrutiny from regulatory bodies and the scientific community.
Blinding, conversely, serves to reduce bias in both treatment administration and outcome assessment. By concealing treatment allocation from participants and investigators, blinding helps maintain objectivity throughout the study process. This is particularly significant in assessments involving subjective outcomes, as it minimizes the risk of expectations influencing results.
Recent statistics underscore the significance of these principles in achieving successful results. For instance, Phase III trials increasingly rely on long-term medical outcomes, such as overall survival, to evaluate the effectiveness of interventions. This shift underscores the need for essential steps in trial design that effectively address the complexities of clinical research.
A case examination titled 'Guidelines for Clinical Trial Design' illustrates the iterative nature of the design process. It highlights that the essential steps in trial design are not merely a checklist but a collaborative effort involving statisticians and research teams. Modifications may be necessary based on various design elements, reinforcing the importance of flexibility and adaptability in planning.
As Deborah D. Stocken noted, 'All authors reviewed and approved the final funder report and manuscript,' emphasizing the collaborative nature of successful oversight.
In 2025, the focus on randomization and blinding remains crucial, as these are considered essential steps in trial design. Expert views consistently support these core principles as vital to the credibility of medical studies. Successful experiments that have utilized these methodologies demonstrate their effectiveness in producing reliable data that can inform medical decisions and regulatory approvals. Additionally, with over 20 years of experience in Medtech, bioaccess® provides extensive research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, ensuring that researchers are well-prepared to navigate the complexities of study design in Latin America.
The training materials created through the GCP for Statisticians project are freely accessible for national and international use, aiming to improve best practices and ensure adherence to regulatory requirements in statistical activities within research. Furthermore, understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial for navigating the regulatory landscape. As medical researchers navigate the complexities of study design, a thorough understanding of these principles will be essential in advancing medical technology and enhancing patient outcomes.
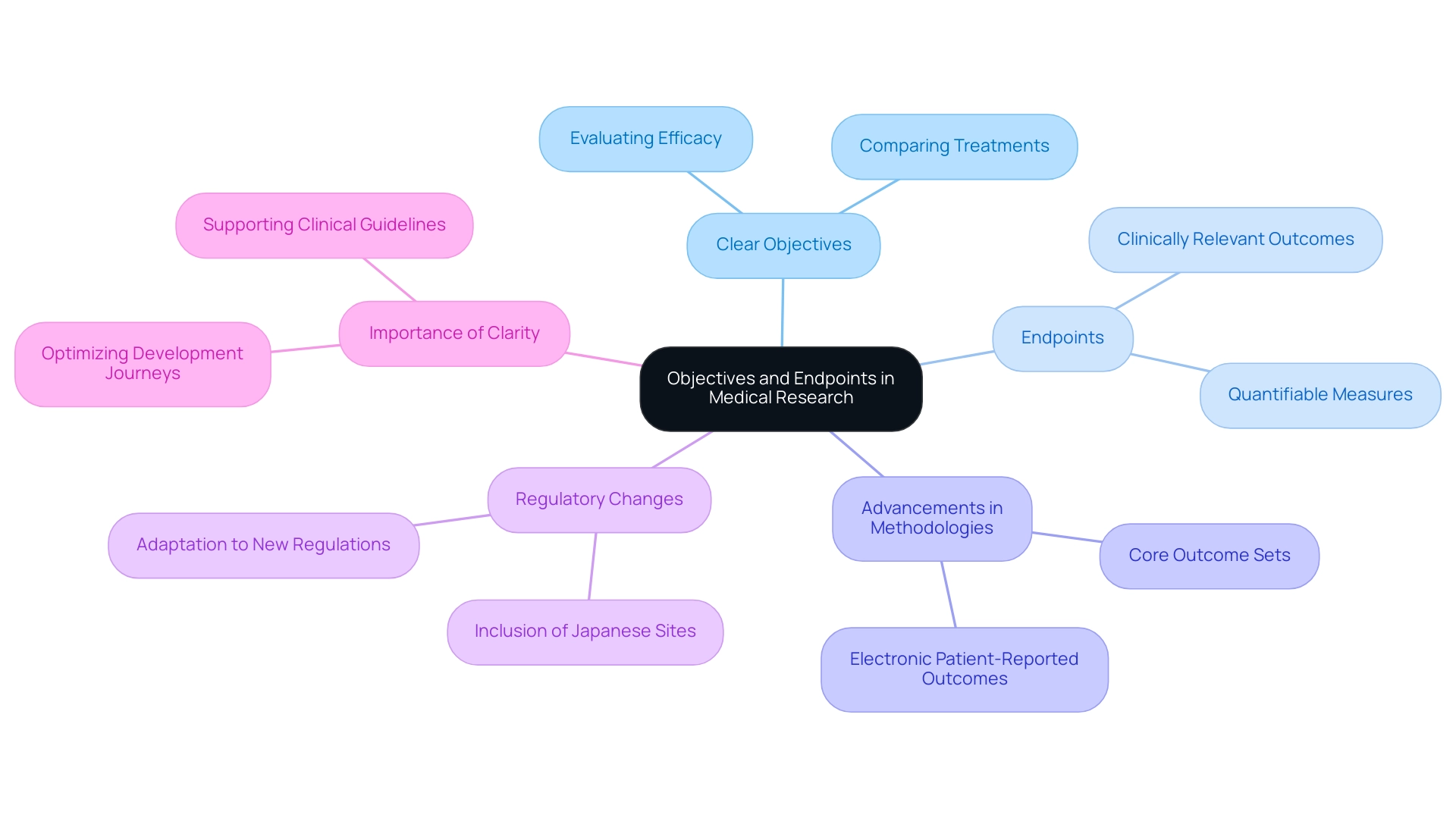
Establishing Clear Objectives and Endpoints
Establishing clear objectives is a fundamental step in trial design, defining the specific aims of research, such as evaluating the efficacy of a new treatment or comparing it against a standard therapy. Objectives must be precise and aligned with the overarching goals of the research. For example, in a study assessing a novel cancer medication, objectives might include determining the drug's impact on overall survival rates or progression-free survival.
Endpoints represent the specific outcomes measured to assess the study's success, and they must be both clinically relevant and quantifiable. The careful selection of appropriate endpoints is crucial; they should reflect meaningful changes in patient health and provide clear evidence of the treatment's effectiveness. By 2025, advancements in electronic patient-reported outcomes (Pros) and the establishment of core outcome sets are expected to significantly enhance data collection, thereby supporting the development of effective therapies.
Furthermore, the importance of precisely assessing results in research studies cannot be overstated. A case analysis on measuring outcomes emphasizes how patient-reported outcomes and biomarker identification play a pivotal role in this process. By ensuring that objectives and endpoints are well-defined, researchers can focus on the essential steps in trial design, leading to results that are not only interpretable but also practical in medical practice.
This case analysis demonstrates that by 2025, advancements in electronic Pros and the establishment of core outcome sets are anticipated to enhance information collection and aid in the creation of effective therapies.
As the landscape of medical research evolves, sponsors are increasingly prioritizing data ownership and transparency, moving towards fully insourced models that demand direct access to live data. This shift underscores the necessity of essential steps in trial design, as clear objectives and endpoints are integral to optimizing development journeys and ensuring commercial success in a competitive market. In this context, bioaccess provides extensive management services for studies, including feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting, all essential for achieving these objectives.
Max Baumann from Treehill Partners cautions that biotech encounters essential business model difficulties as the market grows competitive, stressing the necessity for clarity in objectives. Furthermore, the Japanese government's recent measures to permit Japanese sites to be included in global multi-center Phase III trials without prior Phase I data further illustrate the evolving research trial landscape, highlighting the importance of adapting objectives and endpoints to meet new regulatory environments.
Moreover, as highlighted by Peng Lu, the chief medical officer of Dutch biotech Pharvaris, 'Standardizing the use of specific outcomes and outcome measures for trials will aid in the development of guidelines and future indirect comparisons among interventions.' This perspective reinforces the critical role that well-defined objectives and endpoints play in the broader context of medical research, ultimately contributing to job creation, economic growth, healthcare improvement, and international collaboration.
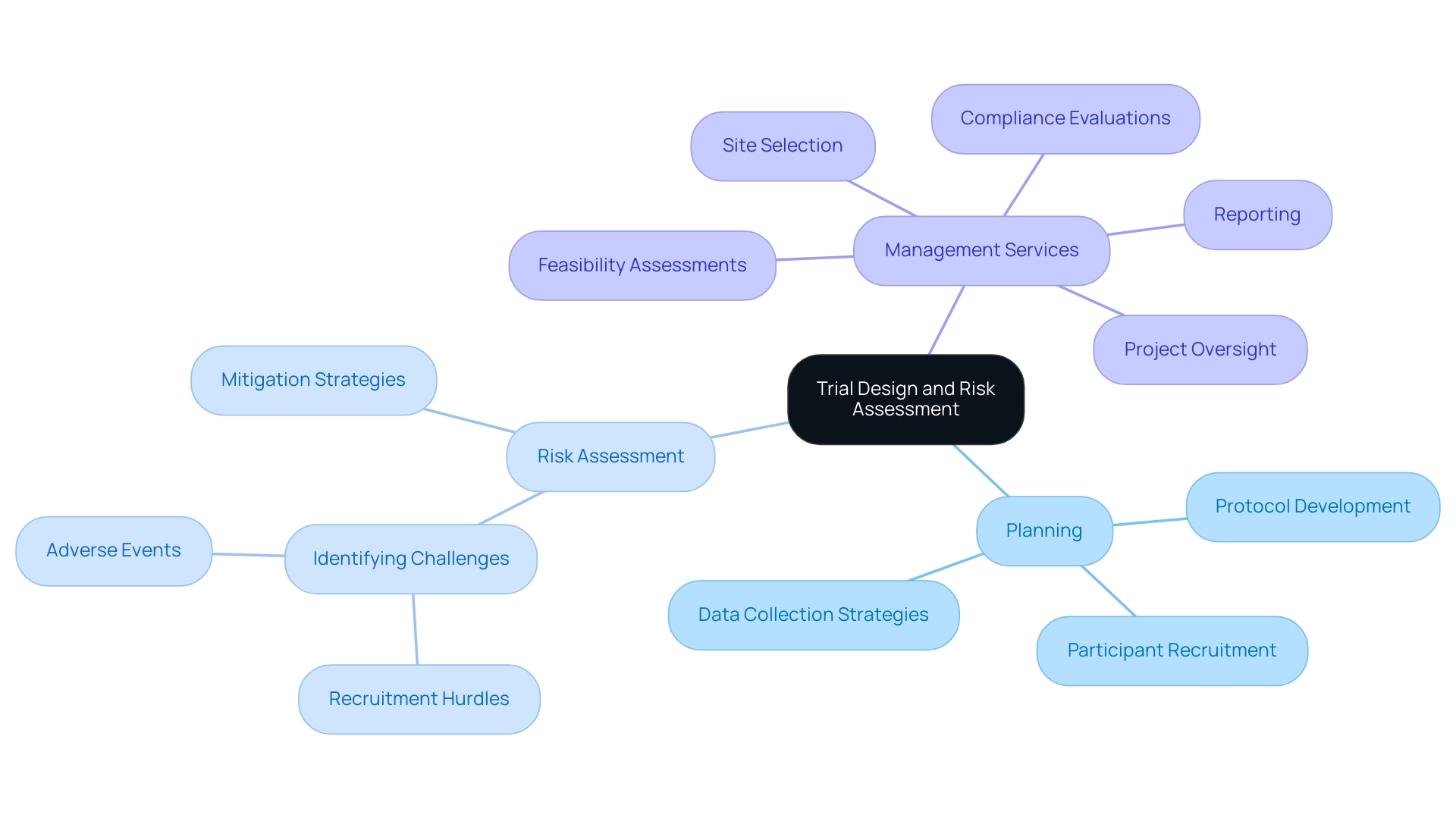
Conducting Rigorous Planning and Risk Assessment
Thorough planning stands as a cornerstone in trial design, necessitating the creation of a comprehensive protocol that delineates every facet of the trial, from participant recruitment to analysis. As we approach 2025, it is expected that study sponsors will increasingly embrace a core outcome set across diverse study designs, highlighting the imperative for meticulous planning. Effective risk assessment is essential; it must proactively identify potential challenges, such as recruitment hurdles or adverse events, and delineate robust mitigation strategies.
For instance, when enrolling participants from multiple sites, implementing strategies to ensure consistent data collection across all locations is crucial. This proactive stance not only preserves the trial's integrity but also significantly boosts the chances of achieving successful outcomes.
Moreover, the biopharma industry currently faces the challenge of crowded trial end-markets, which complicates recruitment efforts. As Max Baumann, Head of Execution, remarked, "Entering 2025, we still witness biotech encountering essential business model difficulties as end-markets grow increasingly crowded." Statistics reveal that numerous studies struggle to fulfill their enrollment targets, often resulting in delays and escalated costs.
By concentrating on the vital steps in trial design, including rigorous planning and risk assessment, organizations can more adeptly navigate these challenges.
In this context, bioaccess™ offers extensive management services for research studies, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
Their methodologies prioritize detailed protocols and risk mitigation strategies, ensuring that experiments are conducted efficiently and effectively. The collaboration with Caribbean Health Group aims to position Barranquilla as a premier hub for research studies in Latin America, with the backing of Colombia's Minister of Health.
This initiative not only enhances the local healthcare landscape but also fosters job creation and economic growth in the region.
A case analysis titled "Focus on Commercial Outcomes in Drug Development" underscores this point; as the industry pivots towards optimizing development journeys, aligning research results with market needs becomes critical. This alignment is anticipated to elevate the likelihood of successful drug launches, underscoring the indispensable role of detailed protocols and risk mitigation strategies in research. Furthermore, the importance of centralized data management and streamlined procedures cannot be overstated, as these elements enhance efficiency and contribute to the overarching success of research endeavors.
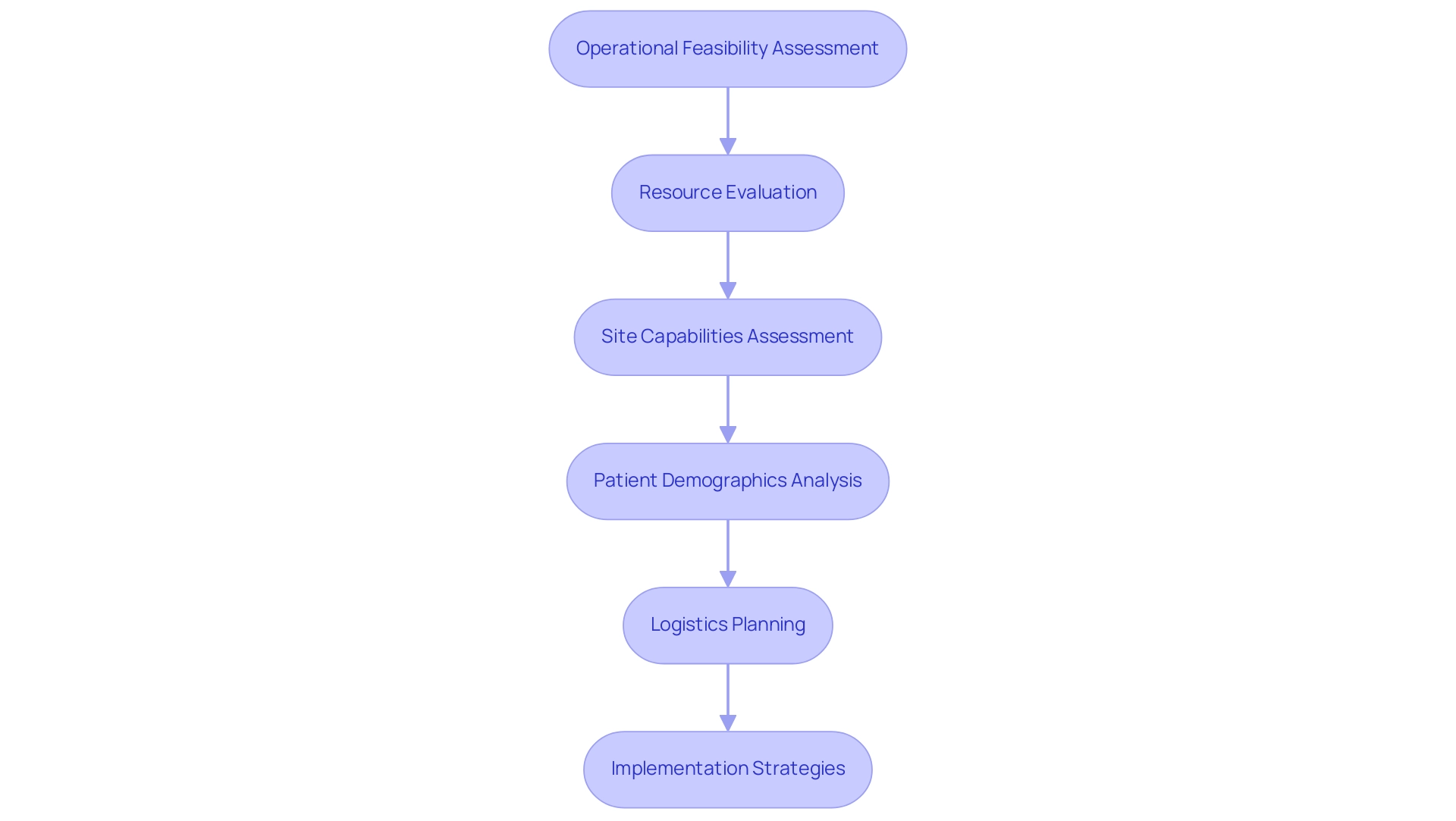
Providing Detailed Assessments and Operational Feasibility
Essential steps in trial design encompass operational feasibility assessments, which are vital for the successful implementation of research studies. These assessments involve a comprehensive evaluation of the necessary resources, timelines, and logistics for the investigation. This process includes a detailed assessment of site capabilities, staff availability, and patient populations. For example, if a study requires specialized imaging techniques, the feasibility assessment must confirm that participating sites possess the required technology and have trained personnel available.
In 2025, the significance of operational viability in research studies is underscored by the need for accurate resource evaluation. Statistics reveal that nearly 30% of clinical studies experience delays due to insufficient resource planning, emphasizing the importance of addressing these factors early in the planning process. By proactively assessing logistics and site capabilities, researchers can ensure that essential steps in trial design are implemented to mitigate risks associated with inadequate study design and enable the project to progress without unnecessary disruptions.
Expert insights indicate that a successful operational feasibility assessment not only evaluates physical resources but also considers the logistical aspects of conducting the study. This includes understanding patient demographics and ensuring that sites can effectively recruit the necessary patient populations. Max Baumann, Head of Execution, notes, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
A case study on the collaboration between bioaccess™ and Caribbean Health Group effectively illustrates this point. This partnership aims to establish Barranquilla as a leading location for medical studies in Latin America, with support from Colombia's Minister of Health. Furthermore, bioaccess® has demonstrated its expertise in managing various types of medical device research, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
This comprehensive approach ensures that essential steps in trial design incorporate thorough and effective operational feasibility assessments. Additionally, a case examination on utilizing advanced analytics in feasibility assessments discusses the challenges researchers encounter when sourcing historical data for medical experiments and emphasizes the importance of consolidating information from various studies to enhance the feasibility evaluation process. Tools such as Anju Software's TA Scan, which analyzes data from over 400,000 research studies globally, enable researchers to access an extensive collection of studies and apply advanced analytics for improved insights, ultimately optimizing the feasibility testing process. Moreover, the integration of electronic health records (EHR) and other data sources is crucial for contemporary research methodologies, ensuring that researchers have comprehensive data to inform their assessments.
By focusing on these critical components, researchers can enhance their operational feasibility evaluations, facilitating smoother execution and increasing the likelihood of success.
Following Content Guidelines and Templates
Adhering to content guidelines and employing standardized templates for clinical studies significantly streamlines the documentation process, ensuring compliance with regulatory standards. The SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines provide a crucial framework, delineating the essential steps in trial design that should be incorporated into a protocol, including objectives, design, and statistical considerations. Research indicates that studies conforming to SPIRIT guidelines exhibit superior quality metrics, with compliance rates linked to enhanced information integrity and expedited review processes by ethics committees and regulatory agencies.
In 2025, the emphasis on organized content standards for research protocols is paramount. As the FDA shifts towards mandating single Institutional Review Board (IRB) reviews, institutions must recalibrate their standard operating procedures and resource allocations accordingly. This transition aligns with the broader trend of risk-oriented methodologies in research, which have proven to yield higher data quality, improved resource efficiency, and shortened project timelines—ultimately accelerating the time to market for new medical devices.
Research studies that adhere to SPIRIT guidelines illustrate the fundamental steps in trial design and highlight the tangible benefits of these practices. For example, bioaccess offers comprehensive management services for studies, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, reporting, and review and feedback on study documents to meet country requirements. These services not only streamline the testing process but also enhance the overall efficiency and effectiveness of research initiatives.
Expert opinions underscore the significance of adhering to content guidelines for research protocols in 2025. Bree Burks, Vice President of Site Strategy at Veeva, notes, "As sponsors reconsider their site engagement approaches in 2025, they will emphasize consistent site technology and standardization across sponsors for all studies." This strategy not only fosters compliance but also enhances collaboration among stakeholders, ultimately contributing to the success of research initiatives, particularly within the context of bioaccess's leadership in Medtech research in Latin America, focusing on innovation and regulatory excellence.
Arranging Protocols for Ease of Reference
Organizing trial protocols for convenience of reference is essential for the success of any research endeavor. A well-structured document employs logical organization, utilizing headings, subheadings, and tables to simplify complex information. For instance, incorporating a table of key milestones allows stakeholders to quickly identify important dates and responsibilities, streamlining communication and enhancing collaboration among team members.
In 2023, the count of registered trials reached an impressive 453,803, reflecting a growing demand for clarity and accessibility in research documentation. This trend underscores the necessity for meticulous organization, as effective documentation not only aids comprehension but also ensures that all team members can readily access the information required to fulfill their roles efficiently.
Expert insights emphasize that a logical organization of research documents significantly contributes to the overall success of studies. As Dipanwita Das, CEO & co-founder, notes, "Last, but certainly not the least is regulatory preparedness. Regulations are getting more complex and more prescriptive and more demanding..." This highlights the importance of staying current with international regulations to ensure a smooth commercialization process.
At bioaccess, our extensive research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
These capabilities are crucial for guaranteeing that research studies are executed effectively and in accordance with local regulations, ultimately resulting in successful outcomes.
Case studies have demonstrated that organizations prioritizing document organization experience enhanced stakeholder access to research information, which is critical in today's fast-paced research environment. For instance, in 2022, China saw a notable rise in medical studies, holding a 27.7% share compared to a decade average of 16.5%. This growth indicates a strong increase in research activities, particularly in non-industry-sponsored studies, emphasizing the global trend towards organized documentation.
Furthermore, the significance of document organization is supported by data showing that well-organized documentation can improve the efficiency of research studies, decreasing the time required for regulatory approvals and accelerating the route to commercialization. Historical milestones in registered studies, such as 100,205 in 2010 and 362,481 in 2020, further illustrate the increasing demand for clarity and accessibility in research documentation. As the landscape of medical research continues to evolve, adopting these essential strategies for structuring research protocols will be crucial for achieving successful outcomes and promoting economic growth through enhanced healthcare and international collaboration.
Understanding Regulatory Requirements for Compliance
Understanding regulatory requirements is a fundamental step in trial design, critical for the successful execution of trials. This necessitates a thorough familiarity with the guidelines established by regulatory authorities such as the FDA and EMA. It encompasses a comprehensive understanding of the necessary documentation, reporting obligations, and ethical considerations vital to the research process. For instance, obtaining informed consent from all participants transcends mere regulatory requirement; it embodies a fundamental ethical obligation that safeguards participant rights.
Moreover, adherence to Good Clinical Practice (GCP) standards is essential for ensuring the integrity of data collection and reporting. In 2025, it is anticipated that approximately 80% of research studies will comply with GCP standards, reflecting an increasing commitment to quality and ethical research practices. This adherence is crucial, as it constitutes a pivotal step in trial design that directly influences the success of medical studies and their acceptance by regulatory agencies.
Additionally, sponsors are required to submit safety reports in a narrative format, which incorporates essential steps in trial design. They can leverage electronic submission methods suggested by the FDA, further streamlining the regulatory process. Extensive research study management services, such as those offered by bioaccess, play a vital role in this landscape. Navigating the complexities of regulatory requirements involves essential steps in trial design, including viability assessments, site selection, compliance evaluations, test setup, import permits, project management, and reporting.
Bioaccess employs specific methodologies, including detailed site evaluations and customized compliance strategies, to ensure that each experiment adheres to the necessary standards. Recent innovations in clinical studies have also focused on enhancing site experiences, significantly improving information entry efficiency and reducing administrative burdens. A case study involving Alcon exemplifies this, as the company has implemented user-friendly systems that monitor site performance, ultimately leading to enhanced support for patient care and more effective studies. This improvement in site experience is closely linked to regulatory adherence, fostering an environment conducive to high-quality information collection.
As the global market for precision oncology approaches $98 billion, understanding the essential steps in trial design for regulatory compliance becomes increasingly crucial. Clinical data leaders are urged to prioritize patient experience and streamline data flows to enhance trial efficiency. By aligning with FDA and EMA guidelines, researchers not only ensure compliance but also position their studies for commercial success.
Max Baumann, Head of Execution at Treehill Partners, underscores this point, stating, 'We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success.' This statement highlights the significance of regulatory compliance in achieving both ethical standards and market viability.
Conclusion
Clinical trial design is increasingly complex, necessitating a strong foundation built on key principles such as hypothesis formulation, randomization, and blinding. These elements are crucial for generating valid and reliable results that meet the rigorous standards of regulatory bodies and the scientific community.
Defining clear objectives and endpoints is equally important. By establishing precise aims and selecting relevant, measurable outcomes, researchers can maintain focus and produce actionable results. Advancements in electronic patient-reported outcomes highlight the need for clarity to ensure trial success and commercial viability.
Rigorous planning and thorough operational feasibility assessments are essential for navigating trial execution challenges. Proactively identifying potential issues and detailing comprehensive protocols enhance the likelihood of successful outcomes while minimizing delays. Compliance with regulatory requirements further ensures ethical standards in clinical research.
Structured content guidelines and organized trial protocols improve communication and collaboration among stakeholders, leading to better data quality and faster commercialization processes.
In summary, integrating well-defined objectives, meticulous planning, and strict regulatory adherence is vital for the success of clinical trials. By embracing these principles, researchers can enhance patient outcomes and drive innovation in healthcare, ultimately shaping the future of clinical research and contributing to global health advancements.
Frequently Asked Questions
What are the essential steps in clinical study design?
The essential steps in clinical study design include formulating a clear hypothesis, implementing randomization to mitigate selection bias, and incorporating blinding to reduce bias in treatment administration and outcome assessment.
Why is randomization important in clinical trials?
Randomization is vital as it ensures that participants are assigned to treatment groups without systematic differences, thereby enhancing the integrity and robustness of the study's findings against scrutiny from regulatory bodies and the scientific community.
How does blinding contribute to a clinical study?
Blinding helps maintain objectivity by concealing treatment allocation from both participants and investigators, which reduces bias, particularly in assessments involving subjective outcomes.
What recent trends are influencing clinical trial design?
Recent trends indicate a shift towards relying on long-term medical outcomes, such as overall survival, in Phase III trials, highlighting the need for effective trial design steps that address the complexities of clinical research.
How does the design process in clinical trials evolve?
The design process is iterative and collaborative, involving statisticians and research teams. Modifications may be necessary based on various design elements, emphasizing the importance of flexibility and adaptability.
What role does collaboration play in clinical trial oversight?
Collaboration is crucial for successful oversight, as noted by Deborah D. Stocken, who emphasized that all authors must review and approve the final funder report and manuscript.
What is the significance of understanding regulatory bodies like INVIMA?
Understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is essential for navigating the regulatory landscape in medical research, particularly as researchers design their studies.
What are the key components of establishing clear objectives in trial design?
Clear objectives define the specific aims of research, such as evaluating the efficacy of a new treatment or comparing it against a standard therapy. They must be precise and aligned with the overarching goals of the research.
Why are endpoints crucial in clinical studies?
Endpoints represent specific outcomes measured to assess the study's success and must be clinically relevant and quantifiable, reflecting meaningful changes in patient health and providing evidence of treatment effectiveness.
How are advancements in electronic patient-reported outcomes expected to impact clinical trials?
Advancements in electronic patient-reported outcomes (Pros) and the establishment of core outcome sets are anticipated to enhance data collection and support the development of effective therapies by 2025.
What challenges do biotech companies face in the evolving research trial landscape?
Biotech companies encounter essential business model difficulties in a competitive market, emphasizing the need for clarity in objectives and adaptability to new regulatory environments.
How does standardizing outcomes and outcome measures benefit medical research?
Standardizing the use of specific outcomes and outcome measures aids in developing guidelines and enables future indirect comparisons among interventions, contributing to improved healthcare and international collaboration.




