Overview
This article addresses the critical trial design challenges within the Medtech sector, underscoring the necessity for innovative study designs that not only meet regulatory requirements but also cater to patient needs. It highlights essential elements such as:
- The alignment of trial objectives with regulatory expectations
- The utilization of advanced technologies for data management
- The incorporation of diverse populations in research
Each of these factors is pivotal for navigating the complexities of Medtech clinical trials and enhancing patient outcomes.
In the evolving landscape of clinical research, Bioaccess plays a vital role in tackling these challenges. By leveraging cutting-edge methodologies and insights, organizations can ensure that their trials are not only compliant but also effective in addressing the diverse needs of the patient population. Collaboration among stakeholders is essential, as it fosters a comprehensive approach to trial design that prioritizes both regulatory adherence and patient-centric strategies.
In conclusion, the importance of collaboration in the Medtech sector cannot be overstated. As the industry continues to evolve, embracing innovative trial designs and fostering partnerships will be crucial for overcoming obstacles and achieving successful clinical outcomes.
Introduction
In the rapidly evolving world of medical technology, clinical trials play a pivotal role in ensuring that innovative devices are both safe and effective for patients. Unlike traditional pharmaceutical trials, Medtech studies face unique challenges that require tailored approaches to trial design, regulatory compliance, and patient recruitment. As the industry shifts towards integrating advanced technologies and real-world evidence, understanding these complexities becomes essential for stakeholders aiming to navigate the intricate landscape of Medtech clinical trials.
From leveraging artificial intelligence to enhance trial efficiency to emphasizing diversity and inclusion in participant selection, the strategies employed today will shape the future of medical devices and their impact on patient care. The significance of collaboration among industry players cannot be overstated, as it is through collective efforts that we can address these challenges and drive innovation forward.
Understanding the Unique Landscape of Medtech Clinical Trials
Trial design challenges for medtech arise from the unique emphasis on innovative medical devices in clinical studies, necessitating distinct designs that significantly differ from those used in pharmaceuticals. A primary trial design challenge encountered in these experiments is the rigorous testing required to establish both the safety and efficacy of devices. This is especially crucial considering that, on average, it takes between 10 to 15 years to develop a new drug, underscoring the urgency for effective study designs in the Medtech sector.
Moreover, the integration of advanced technology into testing protocols presents additional complexities. For instance, the use of real-world evidence has become increasingly vital in supporting claims made by medical device manufacturers. This shift underscores the importance of addressing trial design challenges for medtech, ensuring that study designs not only meet regulatory standards but also reflect the realities of patient experiences and outcomes.
Expert opinions highlight the significance of aligning objectives with regulatory expectations. This alignment is crucial for facilitating a smoother approval process, ultimately leading to quicker access to innovative devices for patients in need. A significant case example from Avantec Vascular demonstrates this point; the company has selected bioaccess® to assist its first-in-human investigation of an innovative vascular device in Latin America.
bioaccess® will assist with site selection, regulatory submissions, and overall study management, ensuring that the research meets both local and international standards.
As the landscape of Medtech continues to evolve, staying informed about the latest statistics and trends in clinical study design is essential. For instance, the restricted number of attempts categorized as 'Expanded Access'—only 888—highlights the challenges in providing treatment options for patients with serious diseases who lack satisfactory alternatives. This statistic serves as a reminder of the essential role that innovative study designs play in overcoming trial design challenges for medtech and enhancing patient care.
In this context, bioaccess® plays a crucial role in connecting innovative Medtech companies with the potential for carrying out research trials in Latin America. Their comprehensive clinical trial management services include feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting. As the Head of Clinical Information Engineering noted, "Traditionally, information management was outsourced to our CRO vendor partners."
Part of the initiative is to bring all our research in-house so that our internal teams can start working on it. They can be more hands-on, allowing us to implement studies internally and take control of our information, ultimately delivering high-quality outcomes for our patients. This method not only improves data management but also aligns with the need for innovative study designs that can adapt to the evolving Medtech landscape.
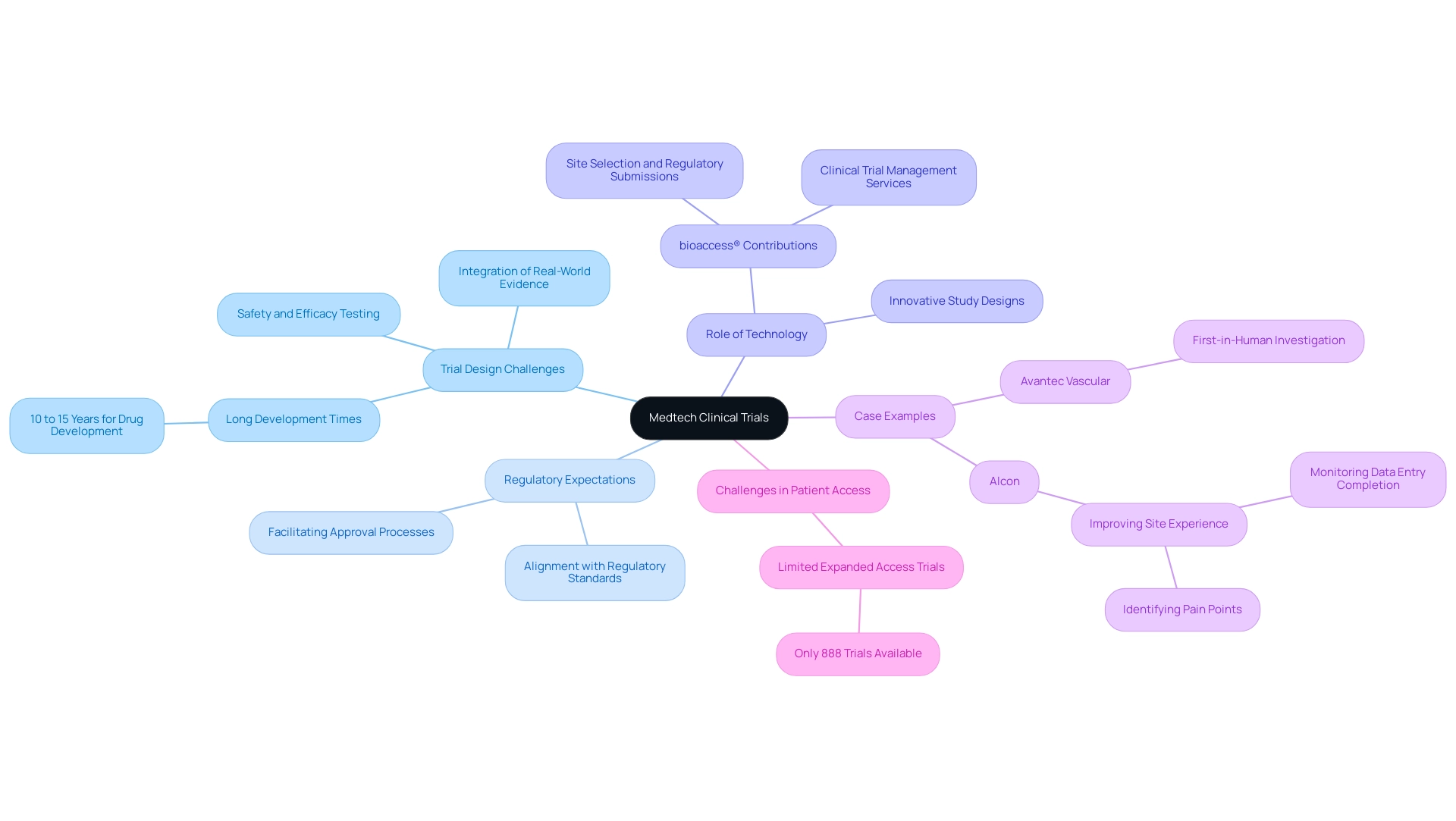
Navigating Regulatory Challenges in Medtech Trials
Medtech companies navigate a complex landscape of regulatory challenges, particularly in adhering to FDA guidelines, CE marking requirements in Europe, and various international regulations. These requirements can vary significantly across regions, complicating the design process. By 2025, it is projected that 44% of Medtech respondents will prioritize offering consumers more personalized health insights, underscoring the critical need for compliance that aligns with evolving market demands.
As the senior vice president of a small German pharma company noted, "We are full of expectations for personalized medicine. With advances in genomics and biomarkers, we can provide patients with more precise treatment options. This not only improves efficacy but also significantly reduces side-effects, allowing patients to have a better treatment experience."
Experts emphasize that early engagement with regulatory bodies is crucial for clarifying expectations and streamlining the approval process. This proactive approach facilitates smoother interactions and aids in navigating the intricacies of post-market surveillance and ongoing compliance, which are vital for ensuring device safety and efficacy after market entry.
At bioaccess, our extensive clinical research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
We also provide review and feedback on documentation to comply with country requirements and reporting on serious and non-serious adverse events. These capabilities are designed to assist Medtech firms in overcoming regulatory obstacles related to trial design challenges and achieving successful results.
A recent case analysis highlights the incorporation of artificial intelligence and machine learning in healthcare research, demonstrating that these technologies can decrease project timelines by up to 30% and expenses by as much as 20%. This efficiency is particularly advantageous in addressing trial design challenges for Medtech within the context of regulatory compliance, as it enhances patient recruitment, retention, and endpoint tracking, ultimately resulting in more effective clinical studies. Additionally, adopting risk-based approaches can help overcome trial design challenges for Medtech by leading to higher data quality, greater resource efficiency, and shorter study timelines, further supporting the overall goal of regulatory compliance.
Furthermore, insights from regulatory bodies indicate that understanding the nuances of CE marking requirements for medical devices in 2025 is essential for Medtech companies. Compliance with these regulations not only ensures market access but also reinforces the commitment to patient safety and product efficacy. As the landscape evolves, staying informed about the latest FDA guidelines and regulatory expectations will be paramount for success in the Medtech sector.
Moreover, with 83% of non-US respondents anticipating significant impacts from the EU's Corporate Sustainability Reporting Directive on their 2025 strategies, Medtech companies must adapt to these changes to maintain compliance and competitiveness. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, underscores the importance of these strategies in transforming lives in Latin America through advanced Medtech solutions.
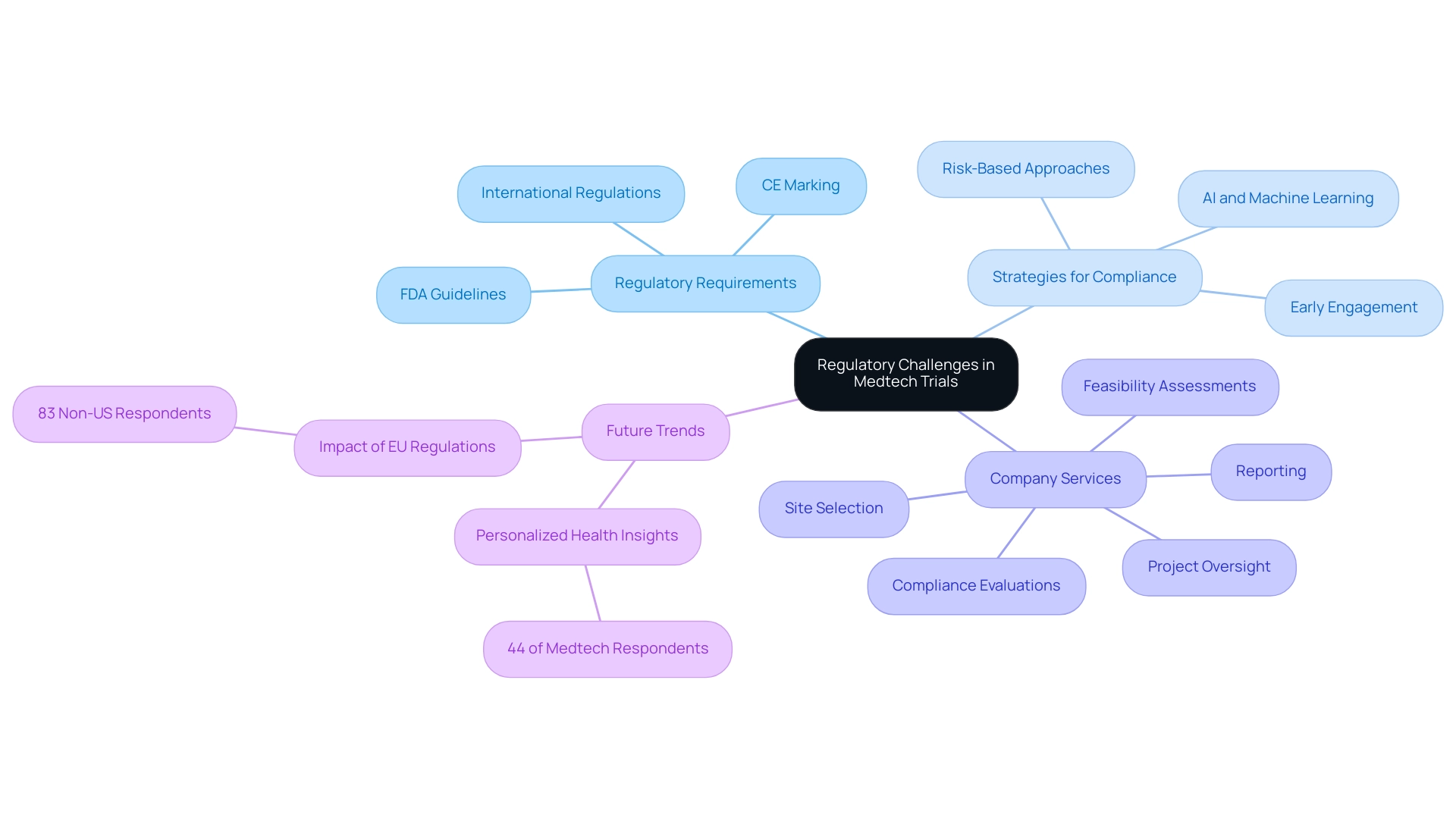
Effective Strategies for Patient Recruitment in Clinical Trials
Recruiting patients for Medtech studies presents significant trial design challenges, particularly due to stringent eligibility criteria and the necessity for informed consent. To navigate these hurdles effectively, leveraging digital platforms has emerged as a pivotal strategy. By 2025, the role of these platforms in patient recruitment is expected to expand significantly, with many organizations utilizing them to enhance outreach efforts.
Collaborating with patient advocacy groups can amplify recruitment initiatives, as these organizations often possess established trust within communities, which is crucial for successful engagement. Social media serves as a powerful tool for increasing awareness about clinical studies, enabling focused messaging that resonates with diverse populations. Experts emphasize the importance of building trust and ensuring that recruitment materials are not only accessible but also culturally sensitive, significantly improving participant engagement. Aditi Shivarkar, a seasoned expert with over 14 years of experience, asserts, "Effective patient recruitment strategies must prioritize trust and accessibility to engage diverse populations successfully."
Furthermore, utilizing analytics to identify potential participants can streamline recruitment processes and enhance timelines. Risk-based methods can lead to improved information quality, enhanced resource efficiency, and reduced project timelines, ultimately accelerating time to market. A recent case involving Alcon illustrates this: by enhancing site experiences—tracking data entry speeds and streamlining query responses—they achieved faster data entry and improved patient care, bolstering the effectiveness of research and enhancing recruitment efforts.
In addition to these strategies, bioaccess provides extensive research study management services, encompassing feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting. These services not only facilitate smoother legal processes but also contribute to the local economy through job creation and healthcare improvements, fostering international collaboration in Medtech.
As the landscape of patient recruitment evolves, addressing trial design challenges for Medtech will be crucial for companies seeking to enhance their research designs and outcomes. Moreover, the reliance on spreadsheets for metadata management has proven difficult for companies striving to scale their operations, underscoring the necessity for MDR solutions that integrate study design, information collection, analysis, and submission. Statistics indicate that North America will dominate the global patient recruitment services market from 2025 to 2034, highlighting the increasing significance of efficient recruitment strategies in this field.
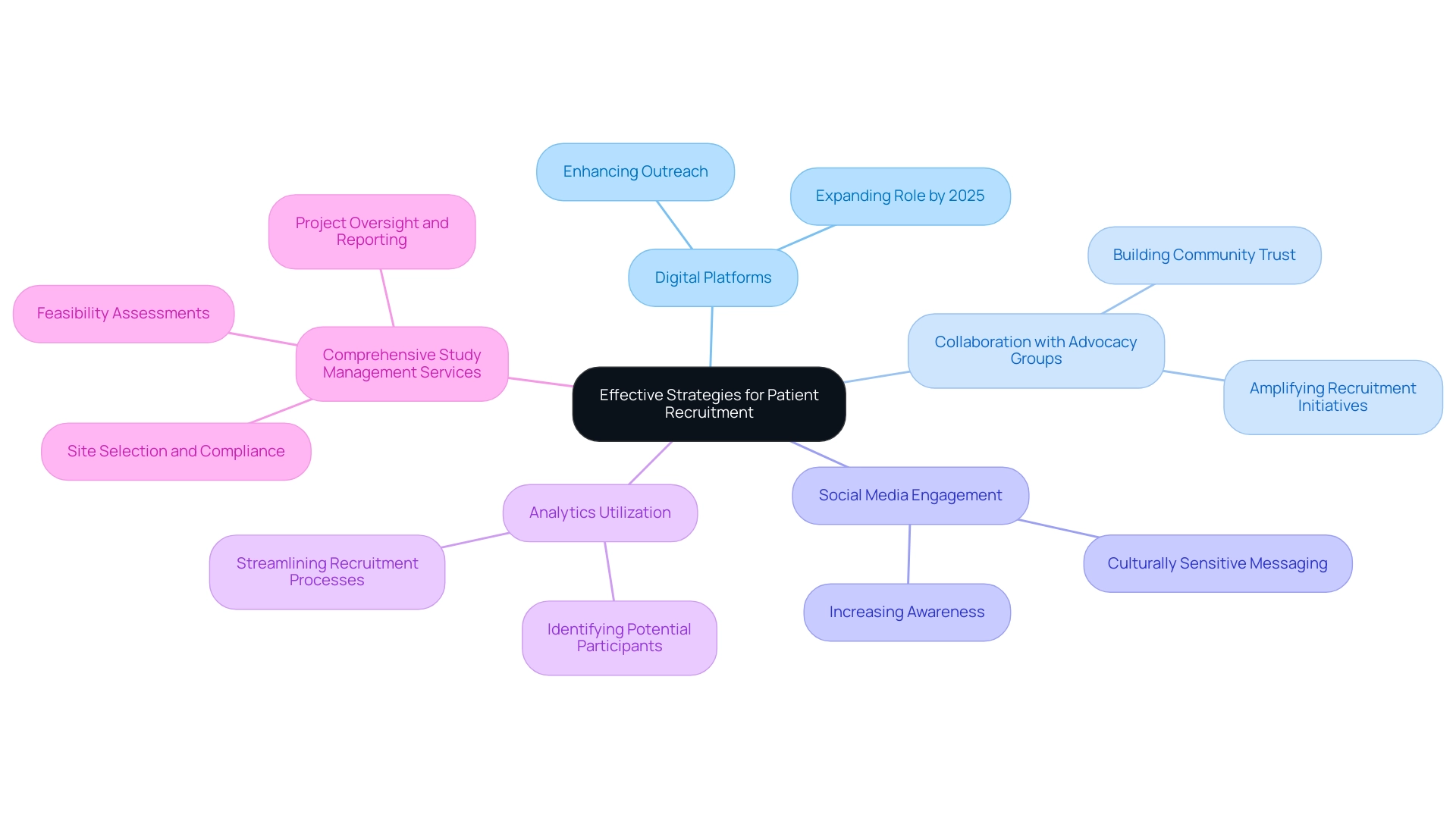
Optimizing Data Management and Collection in Medtech Trials
Effective information management in Medtech trials is essential for overcoming trial design challenges and ensuring the integrity and reliability of clinical outcomes. Central to this process is the integration of electronic information capture (EDC) systems, which streamline data collection and enhance accuracy. These systems are crucial for capturing essential information such as patient demographics, health history, and laboratory test outcomes, thereby enabling comprehensive analysis.
An electronic case report form (eCRF) serves as a cornerstone of this data collection process, ensuring that essential information is systematically recorded.
When collaborating with bioaccess®, you benefit from a team with over 20 years of experience in overseeing research studies across Latin America, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
This expertise ensures that your assessment is not only compliant with local regulations but also optimized for success. bioaccess® employs a personalized strategy designed to address the distinct requirements of every client, offering the adaptability necessary to manage the intricacies of medical device clinical evaluations.
Establishing clear protocols for information collection is essential. Experts suggest that all team members undergo comprehensive training on these protocols to ensure consistency and compliance throughout the process. The adoption of cloud-based platforms can significantly enhance collaboration among research teams, allowing for seamless information sharing and real-time updates.
Real-time oversight has grown more significant in medical studies, with statistics showing that it can decrease information inconsistencies by as much as 30%. This proactive approach not only enhances information quality but also accelerates decision-making processes. Additionally, the FDA's recent draft guidance on Diversity Action Plans stresses the necessity for sponsors to recruit more patients from underrepresented groups, underscoring the significance of diversity in research studies.
Regular audits and compliance checks are essential for preserving information integrity and fulfilling regulatory requirements, ultimately supporting the reliability of trial outcomes. Case studies highlight the trial design challenges for Medtech encountered in implementing EDC systems, particularly the resistance to change among research professionals. However, organizations are overcoming these hurdles by focusing on user-friendly designs, comprehensive training programs, and robust integration capabilities.
Such strategies have led to improved adoption rates of EDC systems, demonstrating their effectiveness in enhancing information management practices.
As Samruddhi Yardi accurately expressed, "Through the dedication of volunteers and the commitment of researchers, medical studies serve as a beacon of hope for a healthier, more resilient future, where medical knowledge continues to grow, and the quality of care consistently enhances for individuals globally." In summary, the significance of electronic information capture systems in Medtech studies cannot be overstated. They not only enable effective information management but also play a crucial role in promoting medical research, ensuring that innovative healthcare devices reach the market more swiftly and safely.
Incorporating Diversity in Clinical Trial Design
Integrating diversity into research design is crucial for ensuring that medical devices are both safe and effective across diverse populations. Experts emphasize the necessity of targeted recruitment strategies that specifically address underrepresented groups, recognizing that these populations often encounter significant barriers to participation, such as transportation challenges and language differences. By designing studies that consider the unique health needs of diverse communities, researchers can collect more comprehensive data, ultimately leading to improved patient outcomes.
Engaging with community leaders and organizations serves as a vital strategy for fostering trust and encouraging participation from diverse groups. This approach not only enhances the inclusivity of medical studies but also aligns with recent regulatory initiatives aimed at increasing diversity in research. For instance, a study highlighted the influence of racial agreement between participants and researchers, revealing that individuals exposed to images of racially matching investigators were significantly more inclined to express interest in joining research studies. This underscores the importance of representation in building trust and enhancing recruitment efforts.
Moreover, the post-pandemic research landscape has witnessed a shift towards utilizing AI-driven tools and automation to streamline recruitment processes, facilitating outreach to underrepresented populations. By leveraging these technologies, Medtech companies such as bioaccess can implement more effective strategies for recruiting diverse participants, ultimately leading to studies that accurately reflect the populations they aim to serve. As the industry evolves, prioritizing diversity in research design will not only fulfill ethical responsibilities but also enhance the scientific credibility of findings.
The rapid vaccine response to COVID-19, which utilized mRNA platforms developed over two decades, exemplifies the significance of innovation in medical studies. Furthermore, as Pierre Chetelat, a Research Associate, noted, "Enhancing inclusivity within studies, along with the implementation of advanced strategies for data transformation, aims to foster trust and bolster the credibility of research findings." Additionally, the case analysis titled "Addressing Disparities for Equitable Research" highlights the lack of diversity in medical experiments and the barriers that prevent minority groups from participating, illustrating a growing recognition of the ethical and scientific importance of fair representation.
Addressing trial design challenges within the Medtech sector enables the industry to ensure that medical studies are more inclusive and representative of the populations they serve. With bioaccess's comprehensive research study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting—researchers are better equipped to navigate these complexities and enhance the overall effectiveness of their investigations.
Leveraging Technology and AI to Enhance Trial Design
The integration of technology and artificial intelligence (AI) is fundamentally transforming clinical study design, presenting significant trial design challenges for Medtech. By utilizing predictive modeling and real-time information analysis, researchers can significantly enhance study efficiency and patient involvement. AI algorithms are increasingly employed to identify optimal study locations, forecast enrollment timelines, and refine patient selection processes, ensuring the right participants are matched with the right studies.
Moreover, digital tools are revolutionizing the way data is collected and monitored. Remote monitoring capabilities alleviate the burden on participants and enhance retention rates, which is vital for the success of clinical studies. As reported by the Tufts Center for the Study of Drug Development, the application of AI and machine learning can reduce study timelines by up to 30% and cut costs by as much as 20%. This efficiency enables more dynamic and responsive experimental designs that can adjust to real-time information.
In 2025, the emphasis on patient experience in study design is paramount. Clinical data leaders are prioritizing strategies that enhance patient engagement, recognizing that a more patient-centric approach can lead to higher participation rates and better outcomes. The global market for precision oncology, anticipated to achieve $98 billion this year, illustrates the increasing significance of innovative research designs that incorporate advanced technologies.
A pertinent case study titled "Elevating Site Preparedness: Trends and Strategies for 2025" emphasizes how the incorporation of AI and machine learning in healthcare studies can enhance operational efficiency and decrease expenses. Furthermore, as highlighted by expert Mathini Ilancheran, who has written more than 35 publications on R&D outsourcing, the enhancement of research processes through technology is crucial for long-term planning and success in the Medtech sector.
Additionally, the FDA's proposed rule for single IRB review necessitates adjustments for some institutions and investigators, emphasizing the need for compliance in the evolving regulatory landscape. As Medtech companies, including bioaccess, continue to explore the potential of AI for predictive modeling, insights into its application will be critical. By employing AI-powered predictive models, researchers can foresee trial design challenges for Medtech and optimize processes, ultimately resulting in more efficient medical studies.
The ongoing evolution of technology addresses trial design challenges for Medtech, supporting operational efficiency and fostering a deeper connection with participants. This progression paves the way for advancements in medical devices that can significantly improve patient lives.
The Role of Post-Market Studies in Medtech Trial Design
Post-market evaluations are integral to the life cycle of medical devices, offering essential insights into their long-term safety and effectiveness. At bioaccess®, we recognize that establishing robust post-market surveillance systems is vital for monitoring device performance in real-world settings. These systems not only help identify rare adverse events but also play a crucial role in informing necessary modifications to device design or usage protocols.
In 2025, the focus on post-market research is underscored by the growing acknowledgment of their significance, with numerous industry leaders advocating for improved monitoring systems. Involving healthcare professionals and patients during these evaluations can greatly enhance information gathering, resulting in better patient safety outcomes. This collaborative approach enables a more thorough understanding of how devices operate outside regulated medical environments. As the life sciences sector progresses, the incorporation of digital advancements and scientific innovations will further enhance the effectiveness of post-market evaluations, ensuring that medical devices not only meet regulatory standards but also fulfill their intended purpose of improving patient care.
The increasing trend among sponsors to consolidate data management internally reflects a desire for greater control over data quality, which is essential for the success of post-market evaluations. This trend aligns with the optimism in the industry, as 68% of executives anticipate revenue increases and 57% predict margin expansions in 2025. Moreover, the FDA's reported 43% year-on-year rise in approvals for MedTech AI devices underscores the evolving landscape of medical devices, highlighting the trial design challenges for MedTech and emphasizing the essential role of post-market surveillance systems as a foundation of effective MedTech research design.
As one industry leader noted, this optimism is driven by strong growth expectations, underscoring the necessity for thorough post-market evaluations to guarantee continued device safety and efficacy. At bioaccess®, we leverage over 20 years of expertise in managing various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring that our clients navigate the complexities of clinical trials in Latin America with confidence.
Conclusion
The complexities of Medtech clinical trials underscore the urgent need for innovative approaches that guarantee the safety and efficacy of medical devices. These trials necessitate unique designs distinct from traditional pharmaceutical studies, emphasizing real-world evidence and advanced technologies. The integration of artificial intelligence and digital tools is revolutionizing trial design, enhancing efficiency, and improving patient recruitment and retention. Strategies that prioritize diversity and inclusion are essential for gathering comprehensive data, ultimately leading to improved health outcomes for all populations.
Navigating the regulatory landscape presents a critical challenge, with early engagement with regulatory bodies proving vital for streamlining approvals. The significance of post-market studies cannot be overlooked, as they provide valuable insights into device performance in real-world settings. With an increasing focus on personalized medicine and patient-centric approaches, the Medtech industry is poised for a transformative shift. By embracing collaboration, leveraging technology, and prioritizing diverse participation, stakeholders can surmount existing challenges and propel innovation forward.
In conclusion, the future of Medtech clinical trials hinges on the ability to adapt to evolving demands and integrate cutting-edge technologies. As the industry continues to progress, fostering a culture of collaboration and inclusivity will be paramount in ensuring that innovative medical devices reach patients safely and effectively, ultimately enhancing the quality of care across diverse populations. A commitment to these principles will shape the next generation of medical technology, paving the way for improved patient outcomes and a healthier future.
Frequently Asked Questions
What are the primary challenges in trial design for medtech compared to pharmaceuticals?
The primary challenges include the rigorous testing required to establish both the safety and efficacy of medical devices, which significantly differs from the trial designs used in pharmaceuticals. It is crucial to create effective study designs that can expedite the approval process, as developing a new drug typically takes 10 to 15 years.
How does the integration of advanced technology impact medtech trial designs?
The integration of advanced technology, such as the use of real-world evidence, adds complexity to trial designs. This shift is vital for supporting claims made by medical device manufacturers and ensuring that study designs meet regulatory standards while reflecting patient experiences and outcomes.
Why is aligning study objectives with regulatory expectations important?
Aligning study objectives with regulatory expectations is essential for facilitating a smoother approval process, which ultimately leads to quicker access to innovative devices for patients in need.
What role does bioaccess® play in medtech clinical trials?
bioaccess® assists medtech companies by providing services such as site selection, regulatory submissions, and overall study management to ensure research meets local and international standards.
What are some key statistics related to medtech trial designs?
One notable statistic is that there were only 888 attempts categorized as 'Expanded Access', highlighting the challenges in providing treatment options for patients with serious diseases who have limited alternatives.
What services does bioaccess® offer for clinical trial management?
bioaccess® offers a range of services, including feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project management, and reporting.
How can artificial intelligence and machine learning benefit medtech trial designs?
The incorporation of artificial intelligence and machine learning can decrease project timelines by up to 30% and expenses by as much as 20%, improving patient recruitment, retention, and endpoint tracking, which leads to more effective clinical studies.
What is the significance of understanding CE marking requirements for medtech companies?
Understanding CE marking requirements is crucial for ensuring market access and reinforcing the commitment to patient safety and product efficacy, especially as regulations evolve.
How will the EU's Corporate Sustainability Reporting Directive impact medtech companies?
The directive is expected to significantly impact the strategies of non-US medtech companies, with 83% anticipating its effects on their operations by 2025, highlighting the need for compliance and adaptability to maintain competitiveness.




