Overview
The article emphasizes the significant advantages of conducting clinical trials in Latin America, particularly highlighting:
- Cost-effectiveness
- Diverse patient populations
- Streamlined regulatory processes
These benefits are substantiated by compelling evidence that showcases:
- Lower operational costs compared to North America and Europe
- The region's rich genetic diversity
- Notable improvements in regulatory frameworks that facilitate expedited study approvals
As a result, Latin America is emerging as an increasingly attractive destination for pharmaceutical research, appealing to stakeholders seeking efficient and effective clinical trial solutions.
Introduction
Latin America is emerging as a hotspot for clinical trials, presenting a wealth of advantages that captivate pharmaceutical companies and research organizations alike. Its cost-effective solutions, diverse patient populations, and streamlined regulatory processes render the region an attractive destination for conducting high-quality research. Organizations like bioaccess® are leading the charge in enhancing clinical trial management, rapidly evolving the landscape and presenting both opportunities and challenges. This article explores the myriad benefits of conducting clinical trials in Latin America, the key factors driving their success, and the innovative trends shaping the future of clinical research in this vibrant region.
The Advantages of Conducting Clinical Trials in Latin America
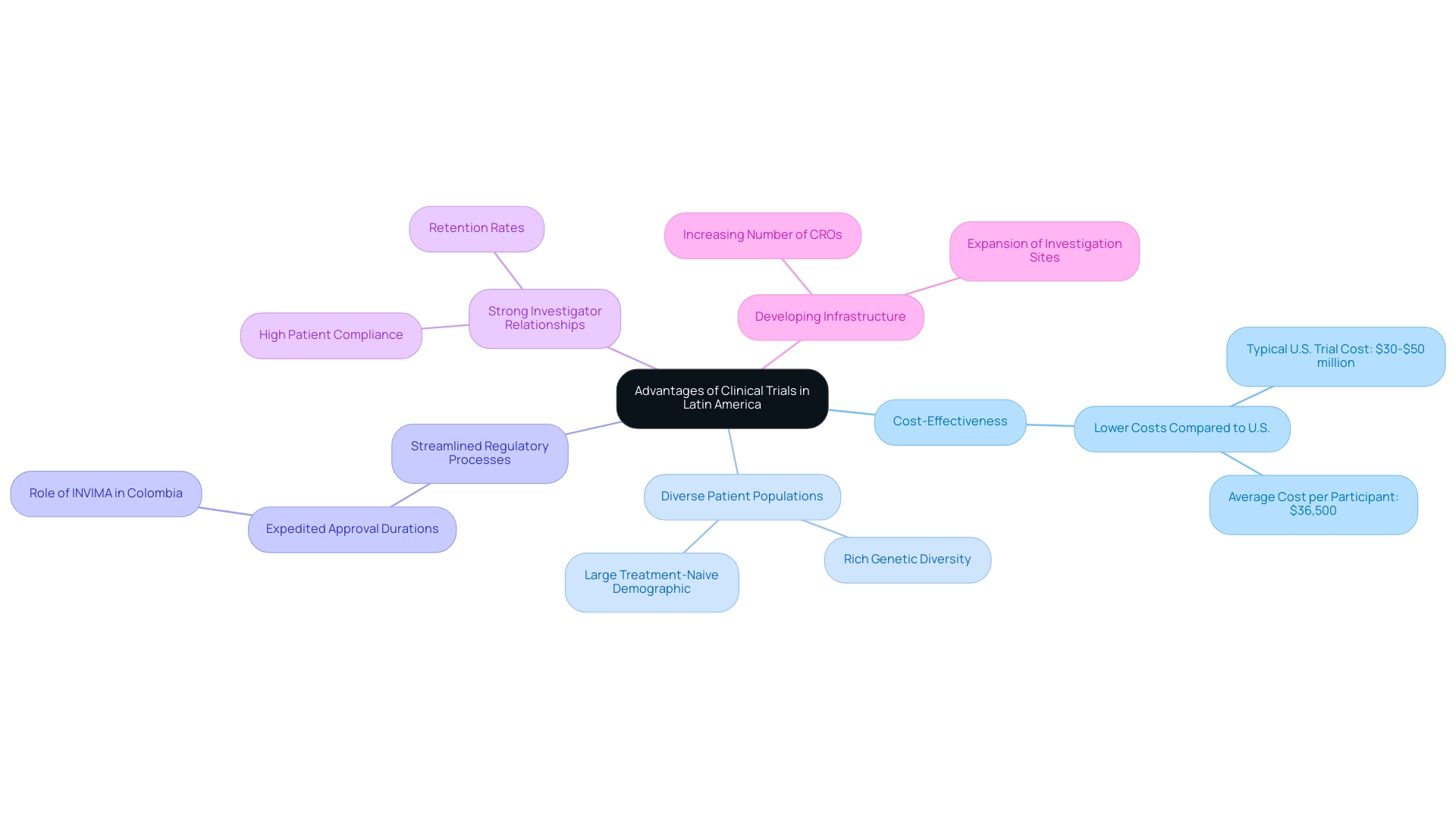
South America presents significant clinical trial benefits, establishing itself as an increasingly attractive location for pharmaceutical firms and investigative organizations to conduct medical studies. The advantages of clinical trials in Latin America are multifaceted:
- Cost-Effectiveness: Conducting clinical studies in Latin America incurs significantly lower expenses compared to North America and Europe. For instance, while the typical cost for a comprehensive medical study in the U.S. ranges from $30 to $50 million, South America provides a more economical alternative, allowing sponsors to optimize their research expenditures.
- Diverse Patient Populations: The region boasts rich genetic diversity and a large treatment-naive patient demographic, which underscores the clinical trial benefits in Latin America. This diversity is crucial for generating robust research data that enhances the applicability of results across various populations.
- Streamlined Regulatory Processes: Numerous nations in Latin America have made strides in simplifying their regulatory frameworks, leading to expedited approval durations for research studies. In Colombia, INVIMA (National Food and Drug Surveillance Institute) plays a pivotal role in overseeing clinical trials, ensuring compliance with health regulations, and accelerating the approval process. This efficiency is essential for companies aiming to hasten their research timelines and bring innovations to market more swiftly.
- Strong Investigator Relationships: The robust investigator relationships in South America are instrumental in fostering high levels of patient compliance and retention, which are vital for the success of medical studies, ensuring that participants remain engaged throughout the study duration.
- Developing Infrastructure: The medical study framework in Latin America is rapidly evolving, showcasing the clinical trial benefits with an increasing number of qualified study organizations (CROs) and investigation sites available to support trials. This growth enhances the capacity to conduct high-quality studies effectively.
Significantly, bioaccess™ has been pivotal in positioning Barranquilla as a key hub for medical evaluations through its collaboration with the Caribbean Health Group, backed by Colombia's Minister of Health. This initiative aims to attract more research projects to the region, thereby demonstrating the clinical trial benefits in Latin America and creating a conducive environment for sponsors while improving the overall landscape of studies.
Moreover, GlobalCare Clinical Studies has partnered with bioaccess™ to enhance ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate. Such metrics underscore the effectiveness of bioaccess®'s comprehensive management services for studies, which encompass feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting. bioaccess® is dedicated to overseeing various forms of medical research, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies (PMCF).
As the medical study landscape in the region evolves, the impact of Medtech research on local economies becomes increasingly significant, fostering job creation, economic growth, healthcare improvement, and international collaboration. For example, the establishment of testing locations has led to expanded job opportunities in healthcare and scientific fields. With Colombia's ambitious science, technology, and innovation strategy for 2022–2031, as highlighted by CEO Julio G. Martinez-Clark, the region is poised to strengthen its role as a key player in global research.
Key Factors Driving Clinical Trial Success in Latin America
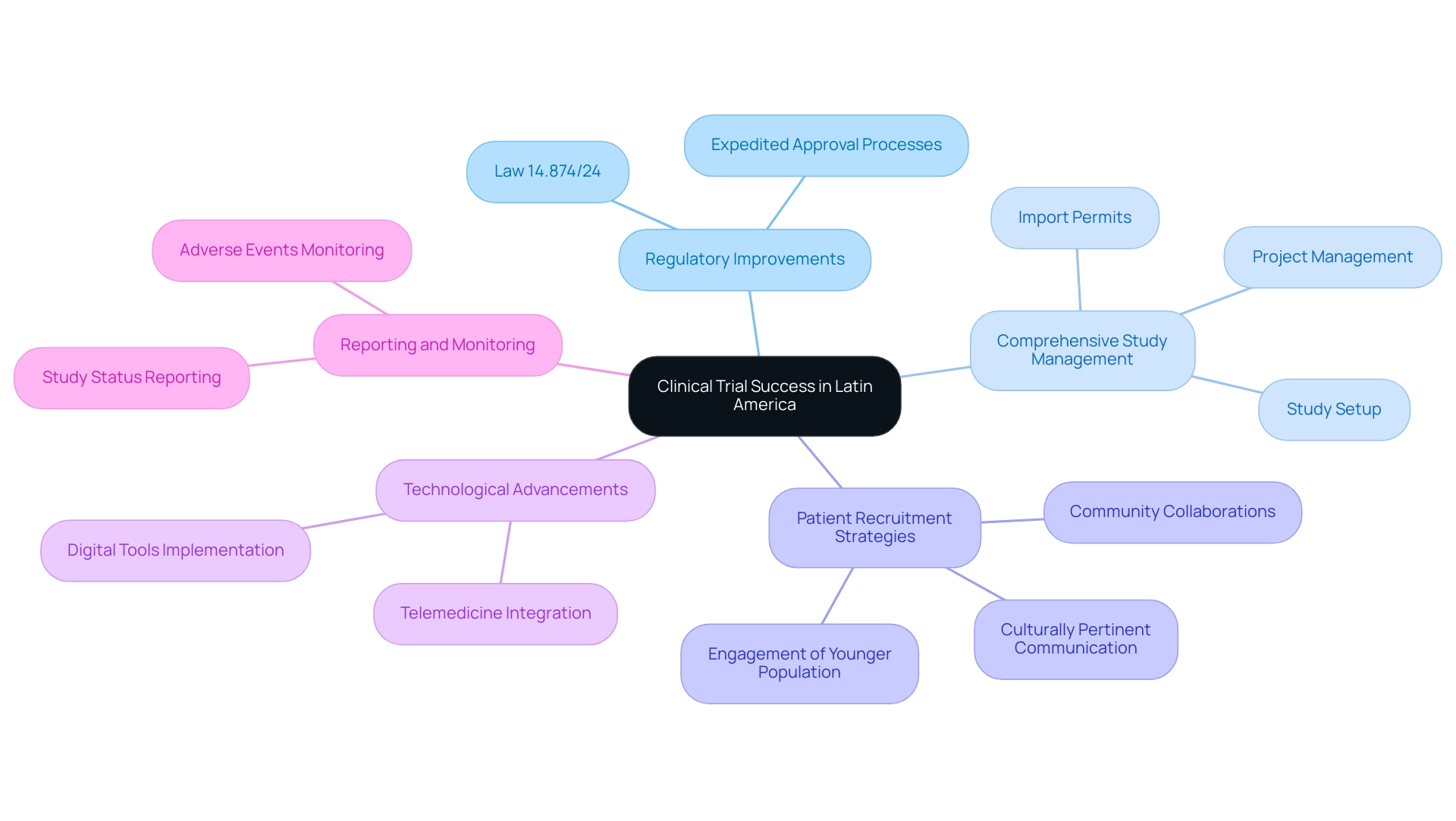
Several key factors contribute to the success of medical studies in Latin America, particularly through the comprehensive services provided by bioaccess.
Countries across the region are actively enhancing their regulatory frameworks, leading to expedited approval processes and improved compliance. This ultimately contributes to the clinical trial benefits in Latin America. For instance, Brazil's recent Law 14.874/24, approved in May 2024, aims to simplify evaluations of research studies, minimizing bureaucratic obstacles and enhancing predictability for researchers. This law represents a significant step towards fostering a more efficient research environment, highlighting the clinical trial benefits in Latin America as it aids in navigating these regulations effectively. The presence of experienced investigators and research professionals in Latin America ensures that studies are conducted with high standards of quality, further contributing to the clinical trial benefits in the region through compliance with established protocols. Bioaccess facilitates feasibility studies and site selection, leveraging local expertise crucial for navigating the unique challenges of the region, thus maximizing the clinical trial benefits in Latin America.
Comprehensive Study Management: Bioaccess provides complete services, including study setup, import permits, and project management. This ensures that all logistical and regulatory aspects of research studies, particularly the clinical trial benefits in Latin America, are addressed efficiently. This comprehensive management allows for smoother operations and adherence to timelines.
Patient Recruitment Strategies: Tailored outreach and engagement initiatives are essential for recruiting diverse patient cohorts. Effective strategies frequently include community collaborations and culturally pertinent communication, which are vital for improving the reliability and applicability of research outcomes, especially regarding the clinical trial benefits in Latin America. With Chile's literacy rate at 95.7%, there exists a well-educated population that can be effectively engaged in research studies, supported by bioaccess's targeted recruitment efforts. A deep understanding of cultural nuances and the establishment of trust within local communities can significantly enhance the clinical trial benefits in Latin America by boosting patient participation and retention rates. This cultural sensitivity is especially crucial in an area where 30% of the population is under 14 years old, indicating a growing demographic that could be essential for future research aimed at younger groups.
Technological Advancements: The implementation of digital tools and telemedicine in research studies has transformed patient access and data gathering procedures. These innovations not only streamline operations but also enhance the overall efficiency of studies, making it easier to gather comprehensive data from a broader patient base. This particularly highlights the clinical trial benefits in Latin America, an area where bioaccess excels through its project management capabilities.
Reporting and Monitoring: Bioaccess places a strong emphasis on reporting study status, inventory, and serious and non-serious adverse events. This ensures compliance with regulatory standards and provides stakeholders with essential insights throughout the research process, underscoring the clinical trial benefits in Latin America.
The Horizon Databook highlights these trends, providing over 1 million market statistics and insights into the research technology and services market. This information can guide companies in making informed decisions and capitalizing on emerging opportunities in collaboration with bioaccess.
Challenges and Considerations in Latin American Clinical Trials
While Latin America presents numerous advantages for clinical trials, including diverse patient populations and cost efficiencies, several challenges must be addressed to ensure successful outcomes:
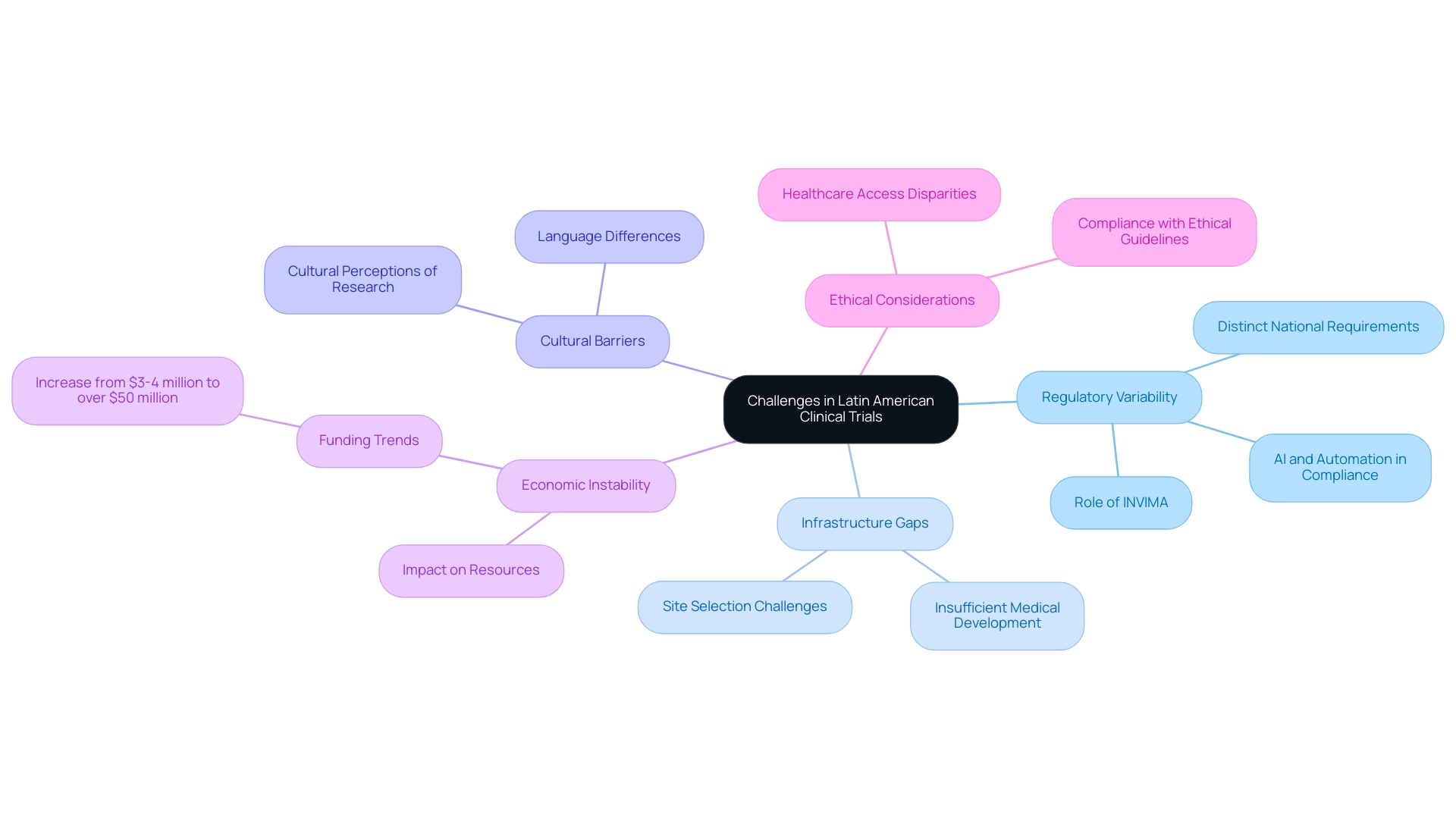
- Regulatory Variability: The regulatory landscape in Latin America is characterized by significant variability across countries. Each nation has distinct regulatory requirements, complicating the execution of multi-country studies. This variability necessitates a thorough understanding of local regulations, particularly the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, which serves as a Level 4 health authority by PAHO/WHO, ensuring medical device oversight and compliance. Bioaccess® provides extensive services, including review and feedback on study documents and setup processes, to navigate these regulatory complexities. The integration of artificial intelligence (AI) and automation is set to transform regulatory affairs, streamlining processes and improving compliance.
- Infrastructure Gaps: Despite the region's potential, certain areas suffer from insufficient medical development infrastructure. This deficiency can hinder the prompt execution of studies, making it crucial for sponsors to identify and partner with sites that have the necessary capabilities to conduct high-quality investigations. Bioaccess® specializes in feasibility studies and site selection to mitigate these challenges.
- Cultural Barriers: Language differences and varying cultural perceptions of medical research can pose challenges in patient recruitment and retention. Understanding and addressing these cultural nuances is vital for fostering trust and ensuring participant engagement throughout the study, a focus area for Bioaccess® in its project management services.
- Economic Instability: Economic fluctuations can significantly affect the funding and resources available for research studies. Over the last ten years, the yearly funding in the research sector in Latin America's Andean Region has increased from $3-4 million to over $50 million. This trend emphasizes the significance of tackling linguistic, cultural, and socio-economic challenges to enhance patient safety and improve the overall quality of medical studies, underscoring the clinical trial benefits in Latin America. Bioaccess®'s expertise in project management and regulatory compliance positions it well to help sponsors navigate these uncertainties, ensuring that projects remain on track despite economic fluctuations.
- Ethical Considerations: Upholding ethical standards is paramount in medical research. Ensuring compliance with ethical guidelines and addressing potential disparities in healthcare access are critical to maintaining the integrity of studies and protecting participant rights. Bioaccess® is committed to navigating these challenges while leveraging the region's unique advantages.
As the area of South America aligns with global best practices, it is set to become an even more significant participant in the global research landscape. According to Sara Tylosky, CEO of Bioaccess®, "As the area of South America aligns with global best practices, it is set to become an even more significant participant in the global research landscape." With more than 15 years of experience in the Medtech sector, Bioaccess® is devoted to offering expedited medical device research services, ensuring thorough management from Early-Feasibility Studies to Post-Market Follow-Up Studies.
Emerging Trends and Innovations in Latin American Clinical Trials
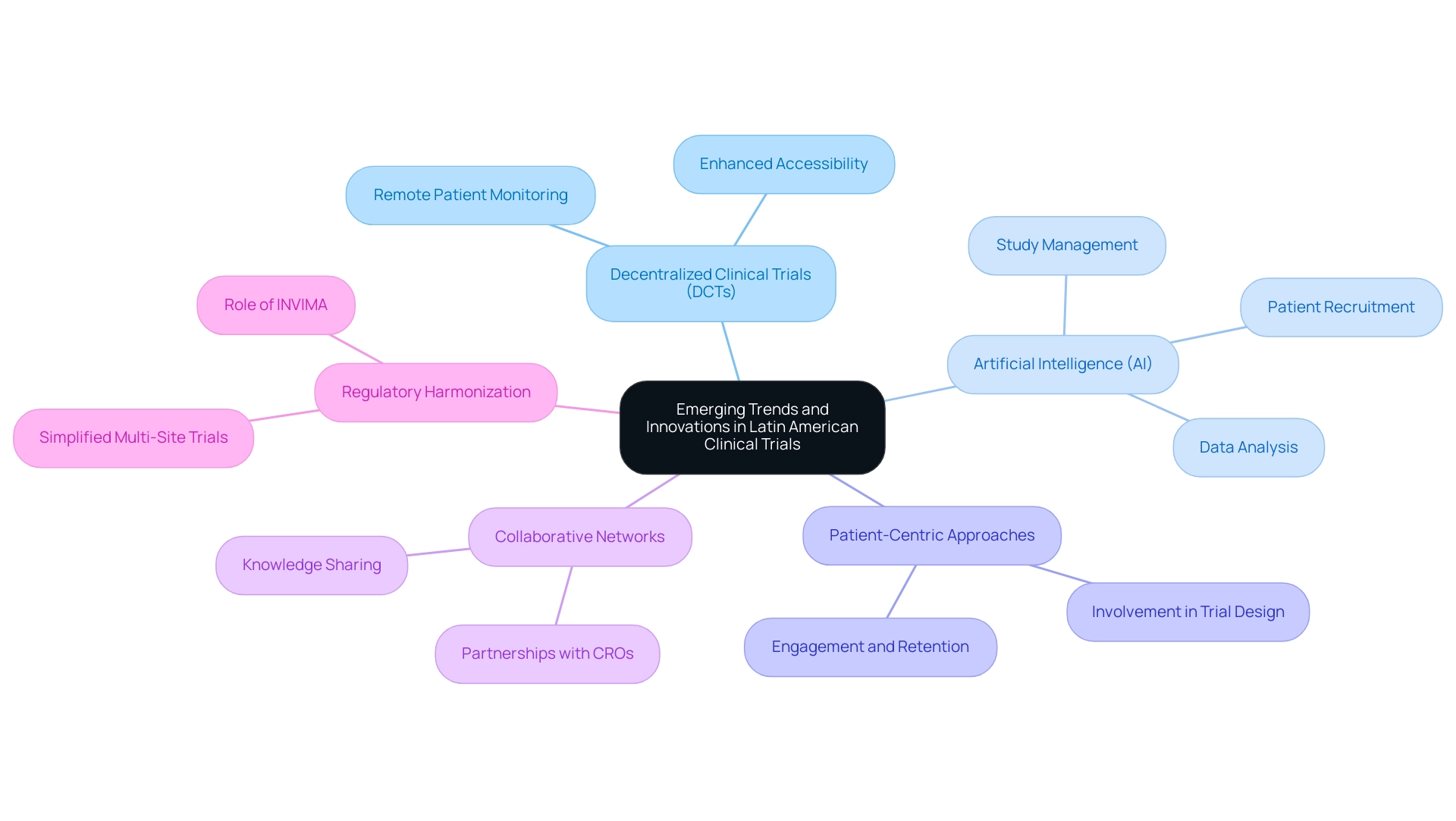
Latin America is currently experiencing a dynamic transformation in research studies, characterized by several key trends and innovations that underscore the clinical trial benefits in the region, particularly in the realm of comprehensive study management services provided by organizations like bioaccess. Their expertise encompasses essential elements such as feasibility and selection of research sites, compliance reviews, setup, import permits, project management, and thorough reporting on study status and adverse events. This holistic approach is vital for navigating the complexities of clinical research in Latin America, especially given their specialization in studies like Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
- Decentralized Clinical Trials (DCTs): The adoption of DCTs is revolutionizing research methodologies, facilitating remote patient monitoring and enhancing accessibility for participants. This approach not only streamlines processes but also broadens the participant pool, rendering studies more inclusive.
- Artificial Intelligence (AI): The integration of AI technologies is reshaping various facets of clinical studies, from patient recruitment to data analysis and study management. By automating routine tasks and providing advanced analytics, AI significantly enhances operational efficiency and decision-making processes.
- Patient-Centric Approaches: There is an escalating emphasis on designing studies that prioritize patient needs and experiences. This patient-centric model bolsters engagement and retention, ultimately leading to more successful outcomes. By actively involving patients in the trial design process, researchers can better address their concerns and preferences.
- Collaborative Networks: The medical research landscape is being enriched by enhanced collaboration among contract research organizations (CROs), academic institutions, and healthcare providers. These partnerships foster innovation and facilitate knowledge sharing, driving advancements in clinical methodologies and practices.
- Regulatory Harmonization: Ongoing efforts to harmonize regulations across South American countries are simplifying the process for sponsors conducting multi-site trials. Notably, INVIMA, the Colombia National Food and Drug Surveillance Institute, plays a critical role in medical device oversight and classification, contributing to the clinical trial benefits in Latin America by ensuring that high standards are met. This regulatory alignment not only enhances the region's competitiveness but also promotes the clinical trial benefits in Latin America by encouraging more international studies.
As part of this evolving landscape, there is a notable trend toward sponsors consolidating data management processes in-house, emphasizing the importance of control and transparency over research data. As the Head of Clinical Data Engineering stated, "Traditionally, data management was outsourced to our CRO vendor partners... We operationalize studies in-house and we are able to take control of our data, and we deliver for our patients with high quality."
Furthermore, effective risk evaluation in medical studies necessitates cross-functional team planning and agreement on terminology to balance structured and flexible discussions.
As the medical research landscape evolves, these trends are anticipated to shape the future of studies in the area, with a projected compound annual growth rate (CAGR) of 12.25% from 2025 to 2030. The strategic advancements in decentralized medical studies, such as efforts by major participants to enhance accessibility, are likely to further bolster the market presence of DCTs in South America. Additionally, the influence of Medtech research on local economies, including job creation and healthcare enhancement, underscores the significance of these studies in promoting international cooperation and economic development.
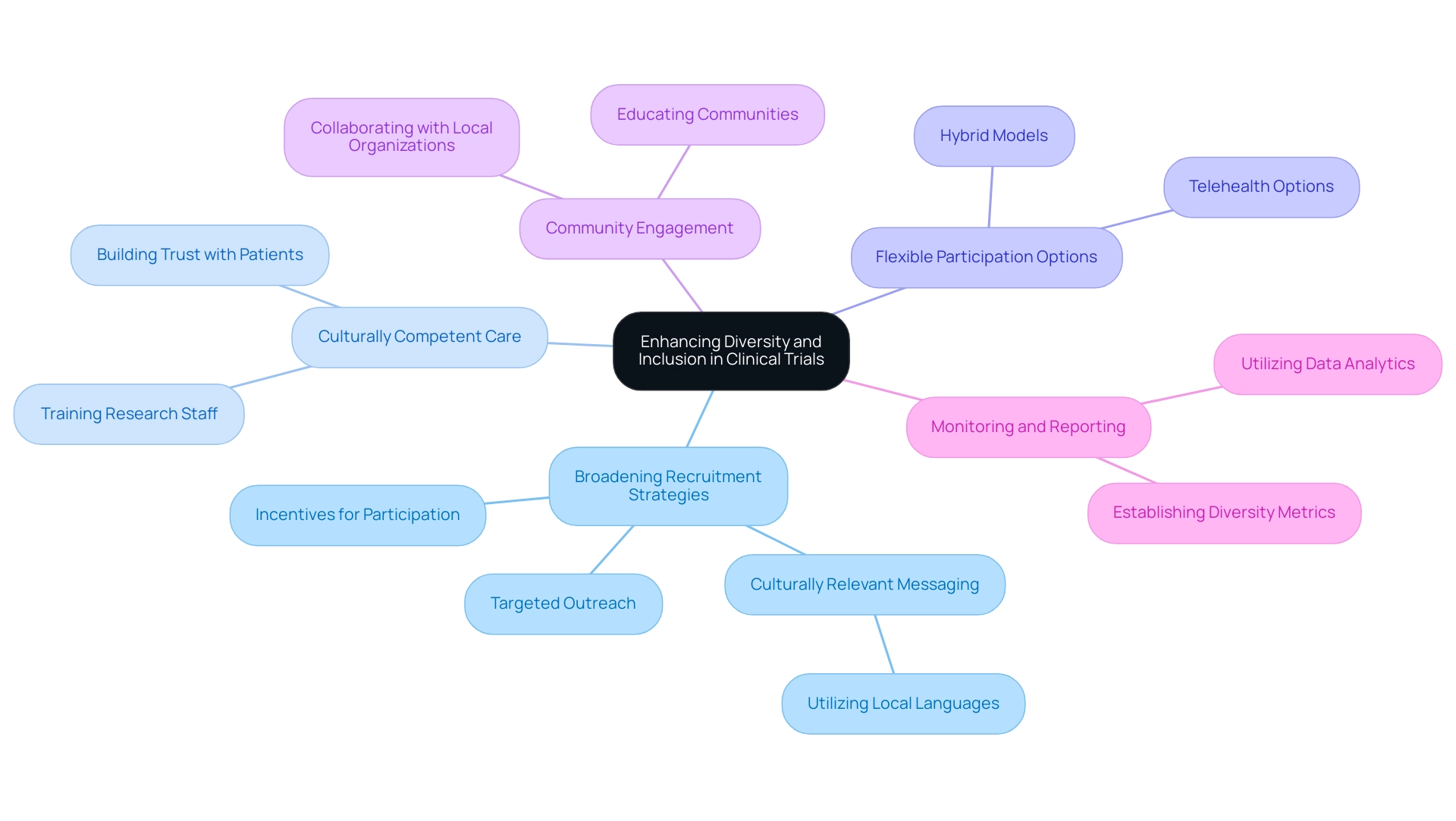
Enhancing Diversity and Inclusion in Clinical Trials
Improving diversity and inclusion in clinical studies is a crucial emphasis in Latin America, particularly in 2025, as the region's clinical trial benefits become increasingly evident within the developing clinical research environment.
Broadening Recruitment Strategies: Targeted outreach initiatives aimed at underrepresented communities are essential for enhancing diversity within study populations. By employing culturally relevant messaging—especially considering Peru's three official languages: Spanish, Quechua, and Aymara—and leveraging local networks, organizations can attract a broader range of participants. Ajay Singh, senior associate dean for postgraduate medical education at Harvard Medical School, emphasizes the importance of providing appropriate incentives for potential trial participants to encourage the recruitment of a diverse population.
Culturally Competent Care: Training research staff in cultural competence is vital for fostering trust and rapport with diverse patient groups. This approach not only enhances participant comfort but also ensures that the unique needs of various populations are understood and respected.
Flexible Participation Options: The introduction of hybrid models for participation, including remote visits and telehealth options, significantly increases accessibility for patients from diverse backgrounds. This flexibility can lead to improved enrollment rates and retention in research trials.
Community Engagement: Collaborating with local organizations and community leaders is essential for effective outreach and education regarding clinical studies. Such partnerships can help clarify the investigative process and encourage participation among communities that may be hesitant due to historical mistrust.
Monitoring and Reporting: Establishing metrics to track diversity in participation is crucial for assessing progress and making necessary adjustments. By regularly reviewing these metrics, organizations like bioaccess can identify gaps and implement strategies to enhance inclusivity. For instance, utilizing data analytics to monitor participant demographics can help tailor recruitment efforts and ensure a representative sample.
Additionally, bioaccess’s comprehensive research management services—including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and detailed reporting—position the company as a key player in advancing research in Latin America. These services directly address the challenges of enhancing diversity, ensuring that trials are designed with inclusivity in mind, which ultimately highlights the clinical trial benefits in Latin America through job creation and improved healthcare outcomes. The varied disease environment in the region, marked by increasing occurrences of conditions like cancer and diabetes, offers unique opportunities for focused research studies.
For example, the elevated rates of particular cancers in nations such as Chile and Peru highlight the potential for studies that directly tackle local health issues. Furthermore, the significant pediatric population indicates a promising market for future drug developments, particularly for seasonal illnesses that can be studied year-round. As South American CROs, including bioaccess®, continue to establish themselves as essential collaborators in the global medical investigation arena, their focus on diversity and inclusion will not only improve the quality of studies but also ensure that the clinical trial benefits in Latin America are fairly distributed across all populations.
Navigating Regulatory Frameworks for Clinical Trials in Latin America
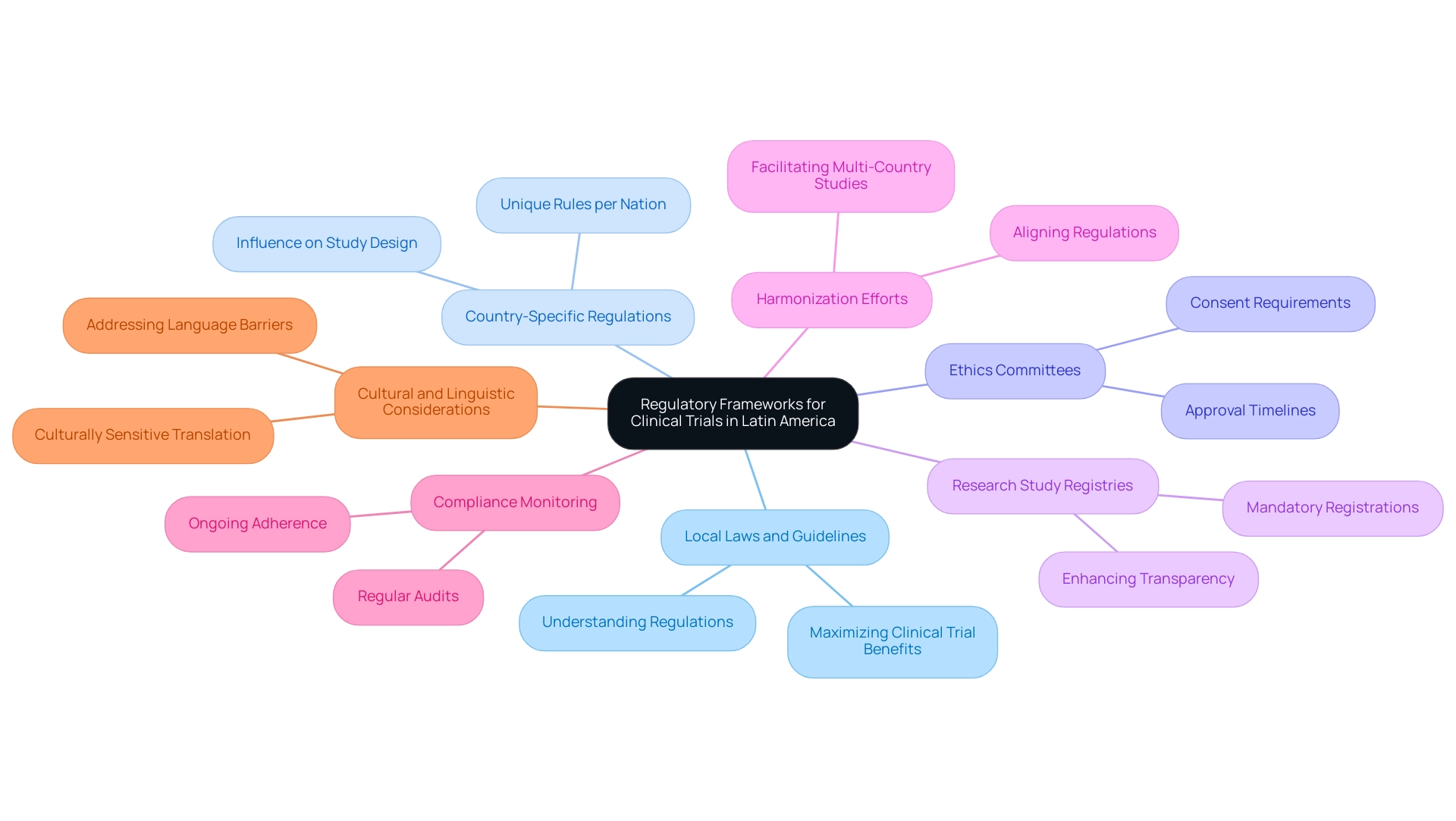
A comprehensive understanding of local laws and guidelines is essential for navigating the regulatory frameworks for medical studies in South America. This knowledge is particularly crucial for maximizing the clinical trial benefits in Latin America, which can significantly influence the success of medical initiatives. Recent partnerships, such as the collaboration between bioaccess™ and Caribbean Health Group, aim to establish Barranquilla as a premier location for medical research in the region, with backing from Colombia's Minister of Health. This exemplifies the strategic collaborations that can enhance access to research opportunities in South America.
Country-Specific Regulations: Each Latin American nation has unique rules governing research studies, leading to variations that can influence study design and execution. Familiarity with these regulations is crucial for compliance and successful results.
Ethics Committees: Securing consent from local ethics committees is a vital step in the research process. Understanding the specific requirements and timelines of these committees is essential for obtaining timely approvals, which can expedite the overall research timeline.
Research Study Registries: Many nations in the region mandate the registration of research studies in national databases. This practice not only enhances transparency and accountability but also fosters trust among participants and stakeholders, aligning with global standards.
Harmonization Efforts: Recent regional initiatives aimed at aligning research regulations across Latin America are facilitating multi-country studies. These efforts can streamline processes and reduce the administrative burden associated with navigating different regulatory environments, thereby enhancing the clinical trial benefits in Latin America.
Compliance Monitoring: Ongoing adherence to regulatory requirements is essential for preserving the integrity of research studies and ensuring participant safety. Regular audits and compliance checks help mitigate risks and uphold ethical standards throughout the research process.
Addressing linguistic and cultural differences is also critical in this context. Language obstacles can present considerable difficulties in research studies, particularly for those initiated outside the region. Applying culturally aware translation methods can enrich the informed consent process and boost participant involvement, ultimately resulting in higher quality research trials.
A case study titled "Language Barriers and Translation in Clinical Research" underscores the importance of accurate translation for logistics, procedures, and ethical treatment of participants. By addressing these language barriers through culturally sensitive translation and understanding local dialects, organizations can mitigate misunderstandings and improve the informed consent process, thus enhancing the clinical trial benefits in Latin America.
Moreover, the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation serves as a pertinent illustration of how regulatory frameworks can influence medical advancement initiatives, expediting approval for promising therapies. By emphasizing these aspects, organizations can effectively bridge the gap between innovative Medtech firms and the clinical trial benefits in Latin America.
The Future of Clinical Trials in Latin America: Opportunities Ahead
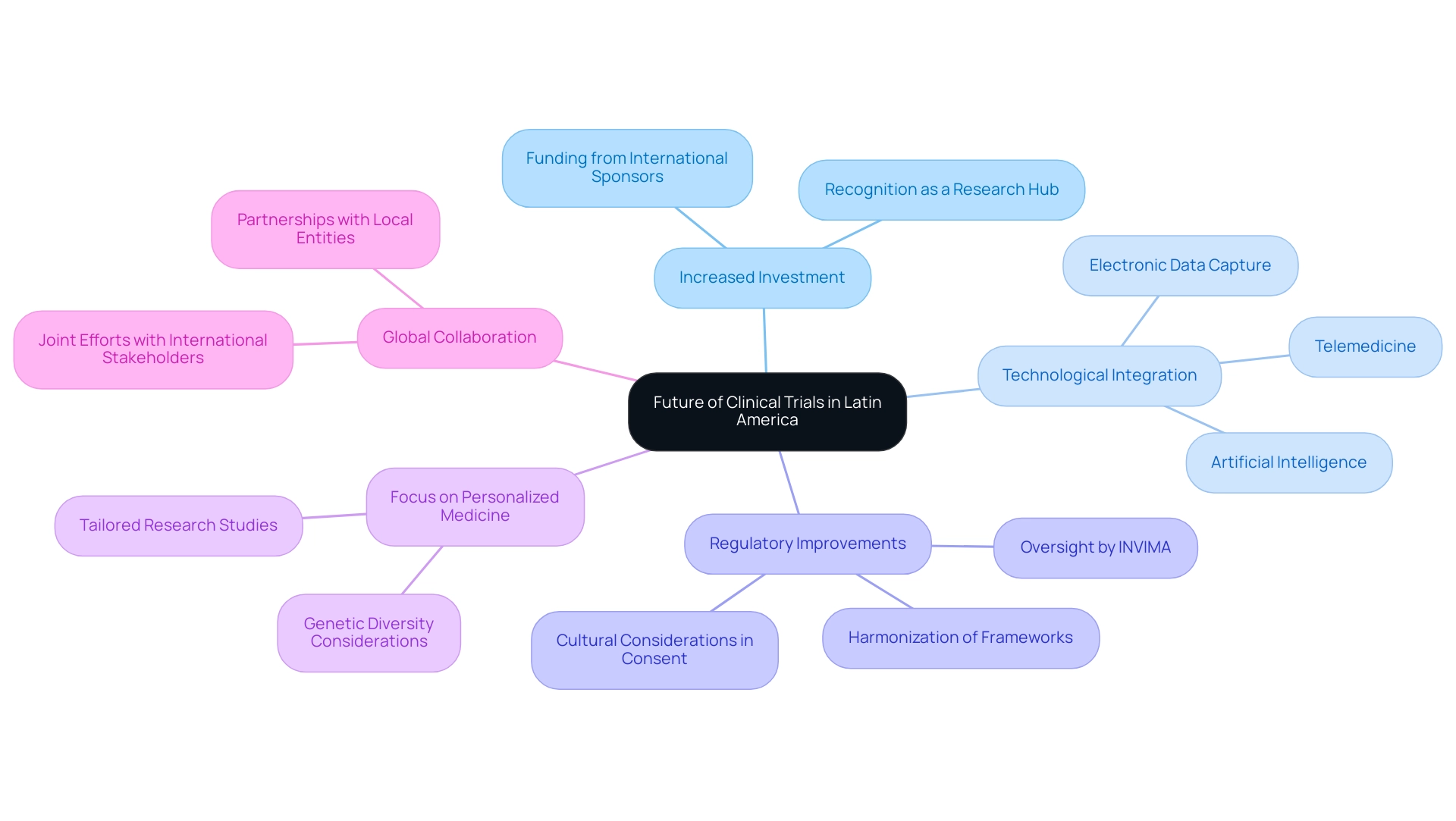
The future of medical studies in South America is poised for substantial expansion, showcasing the clinical trial benefits in Latin America and presenting various opportunities for stakeholders in the medical technology industry. This growth is particularly supported by expert teams like bioaccess®, which focus on comprehensive study management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
- Increased Investment: The region's potential as a hub for medical research is gaining recognition, leading to a rise in funding from international sponsors. This trend is anticipated to continue as more companies seek to leverage the clinical trial benefits in Latin America, as highlighted in the Horizon Databook, which offers comprehensive data and insights into the technology and services market for research in the region.
- Technological Integration: The integration of cutting-edge technologies is set to revolutionize trial efficiency and enhance patient engagement. Innovations such as telemedicine, electronic data capture, and artificial intelligence are streamlining processes and improving the overall effectiveness of clinical studies.
- Regulatory Improvements: Efforts to harmonize and simplify regulatory frameworks across Latin American countries, including oversight by INVIMA, are currently underway. These enhancements will facilitate smoother operational processes, making it easier for companies to navigate the complexities of conducting research in the region. However, cultural differences complicate the informed consent process for clinical trial benefits in Latin America, as patients may accept physician recommendations without question, necessitating customized approaches in research studies.
- Focus on Personalized Medicine: With a growing emphasis on personalized medicine, there is an increasing demand for research studies that consider the genetic diversity of the population. This shift will drive the need for more tailored studies, ultimately enhancing patient outcomes and underscoring the clinical trial benefits in Latin America.
- Global Collaboration: Strengthening partnerships between local entities and international stakeholders is crucial for fostering innovation. Joint efforts will not only improve the quality of medical studies but also highlight the clinical trial benefits in Latin America, establishing the region as a competitive participant in the global health study arena. As the pediatric population in Latin America continues to grow, with 30% under the age of 14, the clinical trial benefits in Latin America create a burgeoning market for new drug development targeting both young and elderly patients. This demographic shift emphasizes the importance of adapting medical studies to meet the needs of diverse populations, further reinforcing the region's position in the future of medical research. As Catherine Gregor, Chief Clinical Trials Officer, noted, 'A confluence of political, technological, and economic forces will shape the clinical trial benefits in Latin America and the ecosystem in 2025.
Conclusion
Latin America stands at the forefront of clinical trial innovation, presenting a compelling array of advantages that are transforming the landscape of medical research. The region's cost-effectiveness, diverse patient populations, and streamlined regulatory processes collectively position it as an attractive destination for pharmaceutical companies and research organizations. With key players like bioaccess® leading the charge, these factors are not only driving the success of clinical trials but also enhancing the overall quality of research conducted in Latin America.
While the growth of clinical trials in this region is accompanied by challenges, the commitment to addressing regulatory variability, infrastructure gaps, and cultural barriers is paving the way for a more robust clinical research environment. Organizations focusing on enhancing diversity and inclusion are improving patient engagement and ensuring that the benefits of medical advancements reach all segments of the population.
Looking ahead, the future of clinical trials in Latin America is bright, characterized by increased investment, technological integration, and a focus on personalized medicine. As regulatory harmonization efforts continue to progress, the region is poised to become an even more influential player in the global clinical research arena. By leveraging its unique advantages and fostering international collaborations, Latin America is set to play a critical role in shaping the future of clinical trials, ultimately driving advancements in healthcare and improving patient outcomes across diverse populations.
Frequently Asked Questions
What are the main advantages of conducting clinical trials in South America?
The main advantages include cost-effectiveness, diverse patient populations, streamlined regulatory processes, strong investigator relationships, and developing infrastructure.
How does the cost of clinical trials in South America compare to North America and Europe?
Clinical studies in South America are significantly less expensive, with costs being much lower than the typical $30 to $50 million range for comprehensive medical studies in the U.S.
Why is the diversity of patient populations important for clinical trials in Latin America?
The region has rich genetic diversity and a large treatment-naive demographic, which enhances the applicability of research data across various populations.
How have regulatory processes in Latin America improved for clinical trials?
Many countries have simplified their regulatory frameworks, resulting in expedited approval durations for research studies, as exemplified by Colombia's INVIMA.
What role do strong investigator relationships play in the success of clinical trials?
Strong relationships with investigators help ensure high levels of patient compliance and retention, which are crucial for the success of medical studies.
How is the infrastructure for clinical trials evolving in Latin America?
The region is seeing an increase in qualified study organizations (CROs) and investigation sites, enhancing the capacity to conduct high-quality studies effectively.
What initiatives are being taken to make Barranquilla a hub for medical evaluations?
bioaccess™ collaborates with the Caribbean Health Group and Colombia's Minister of Health to attract more research projects to the region.
What impact does Medtech research have on local economies in Latin America?
Medtech research fosters job creation, economic growth, healthcare improvement, and international collaboration.
What comprehensive services does bioaccess provide for clinical trials?
bioaccess offers services including feasibility assessments, site selection, compliance reviews, project management, and reporting.
How does bioaccess enhance patient recruitment for clinical trials?
Tailored outreach and culturally relevant communication strategies are used to engage diverse patient cohorts effectively.
What technological advancements are being utilized in clinical trials in Latin America?
The implementation of digital tools and telemedicine has improved patient access and data gathering processes, enhancing study efficiency.
How does bioaccess ensure compliance and monitoring during clinical trials?
bioaccess emphasizes detailed reporting on study status, inventory, and adverse events to ensure compliance with regulatory standards.




