Overview
Understanding Dominican Republic clinical trials is crucial for participants as it outlines their rights, the phases of trials, and the importance of diversity in research. The article emphasizes that informed consent and ethical oversight are essential for participant safety, while diverse representation enhances treatment efficacy and relevance across different demographic groups, ultimately contributing to improved healthcare outcomes.
Introduction
Clinical trials serve as the backbone of medical innovation, meticulously designed to assess the safety and effectiveness of new interventions ranging from pharmaceuticals to advanced therapies. As the landscape of healthcare evolves, these trials have become increasingly vital, with a remarkable 94% of interventional studies reporting their findings as of May 2023. This statistic not only highlights the importance of clinical trials in advancing medical knowledge but also reflects the collaborative efforts of researchers and institutions to enhance patient outcomes.
However, the journey from concept to clinical application is fraught with challenges, particularly in regions like Latin America, where regulatory complexities and resource limitations can impede progress.
This article delves into the multifaceted world of clinical trials, exploring:
- Their purpose
- The rights of participants
- The phases of research
- The critical role of diversity and ethics in ensuring that these studies yield meaningful and applicable results for all populations.
Introduction to Clinical Trials: Understanding Their Purpose and Importance
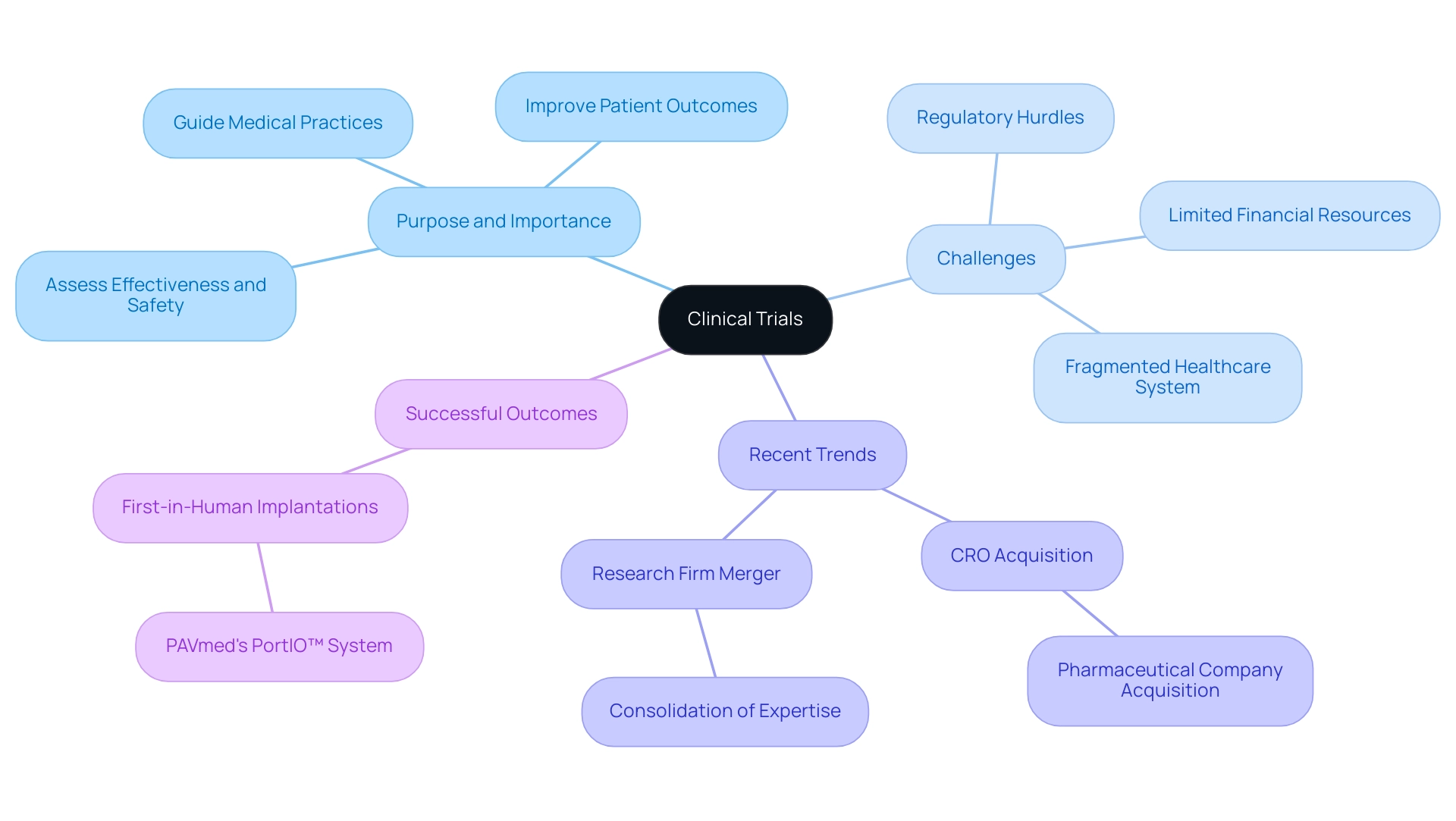
Clinical experiments are meticulously crafted research projects aimed at assessing the effectiveness and safety of new medical interventions, including drugs, therapies, and procedures. As of May 2023, an impressive 94% of interventional research studies have posted their results, underscoring the vital role these experiments play in advancing medical knowledge. Dr. Ting Ye, the lead researcher and main contact for various projects, states, 'Clinical studies are essential for ensuring that new treatments are safe and effective, ultimately leading to improved patient outcomes.'
These experiments offer crucial empirical information that guides medical practices and fosters advancements in treatment alternatives. However, Medtech companies in Latin America face significant challenges, including:
- Regulatory hurdles
- Limited financial resources
- A fragmented healthcare system that complicates collaboration
The ongoing partnership between bioaccess® and Caribbean Health Group aims to position Barranquilla as a leading destination for medical studies in Latin America, supported by Colombia's Minister of Health.
This partnership exemplifies efforts to bridge the existing gaps in medical research and innovation. Moreover, involvement in research studies is essential, as it enables scientists to examine how new therapies function among diverse groups. Recent trends, such as:
- The acquisition of a contract research organization (CRO) by a pharmaceutical company in September 2023
- The merger of two research firms in December 2023
further highlight the evolving landscape of studies and their impact on medical advancements.
Successful outcomes, such as the first-in-human implantations of PAVmed's PortIO™ Intraosseous Infusion System in Colombia, demonstrate the potential for effective therapies tailored to meet the specific needs of patients, thereby improving overall health outcomes.
Your Rights as a Clinical Trial Participant: What You Need to Know
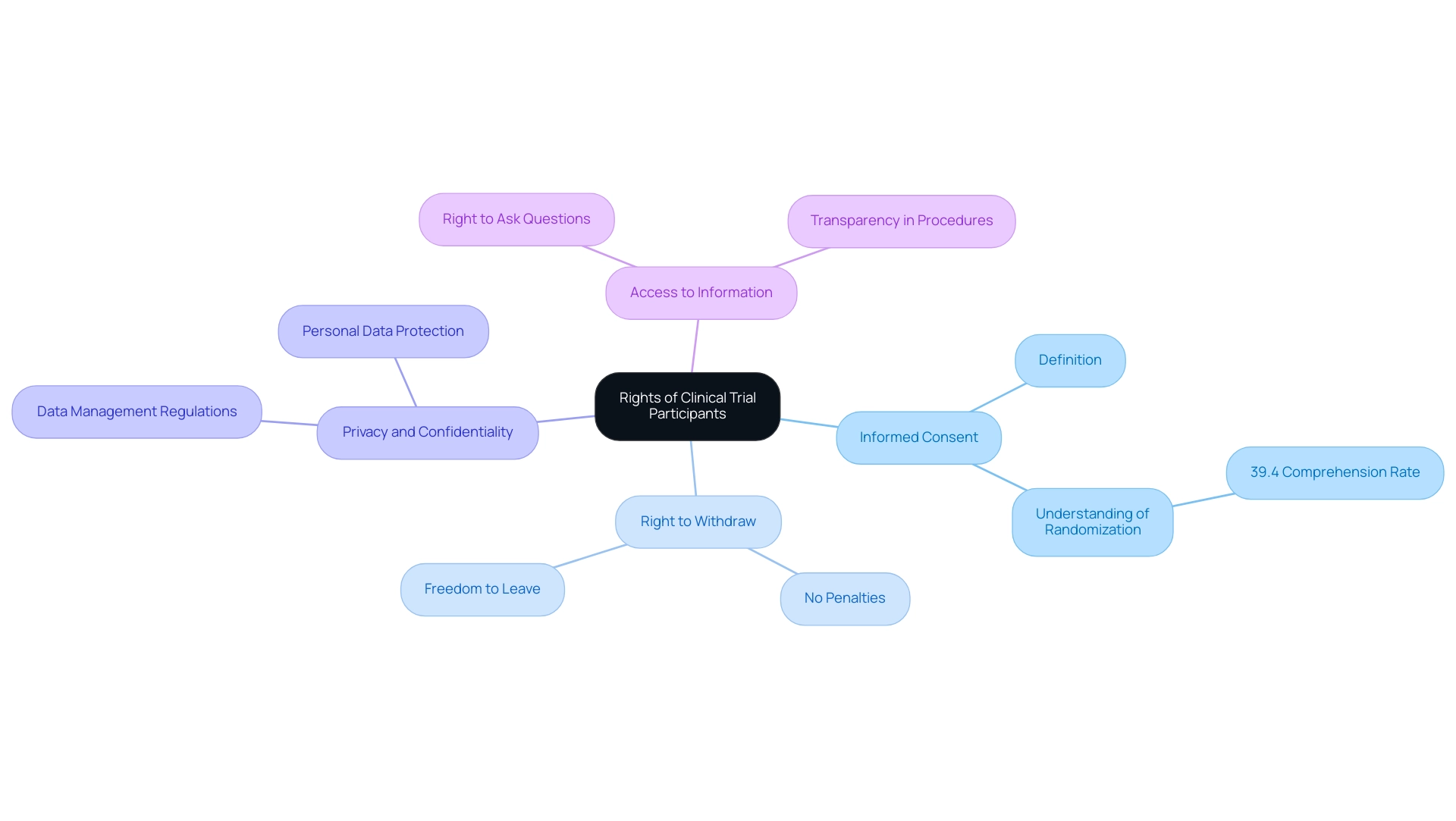
Participants in clinical studies possess essential rights that safeguard their well-being and ensure ethical treatment throughout the research process. Foremost among these rights is the right to informed consent, which mandates that individuals receive comprehensive information regarding the study’s purpose, procedures, potential risks, and benefits before agreeing to participate. Current statistics reveal that understanding of randomization is notably low, with only 39.4% of individuals grasping this crucial aspect, underscoring the need for clearer communication.
This is particularly relevant considering the findings by McNally T et al., who evaluated parents' comprehension of a randomized controlled study, emphasizing the difficulties in communicating complex information effectively. Participants have the unequivocal right to withdraw from the study at any time without facing penalties, and they are entitled to seek answers to any questions they may have regarding the research. Furthermore, individual privacy and confidentiality are paramount, necessitating the management of personal data in strict accordance with applicable regulations.
As Mike Hennessy Jr. aptly notes,
This November issue of ACT explores those technological innovations in clinical study recruitment and data collection and management that are being geared toward empowering the research community's most important asset—the patient.
This focus on patient empowerment is especially significant as we approach 2024, where informed consent procedures continue to develop to improve transparency and engagement. Furthermore, insights from the case study titled 'A Singular Root Cause Undermines Clinical Success' illustrate that addressing fundamental issues is essential for improving outcomes.
Comprehending these rights is essential for individuals to make informed choices about their engagement in clinical studies.
Navigating the Phases of Clinical Trials: What Each Stage Entails
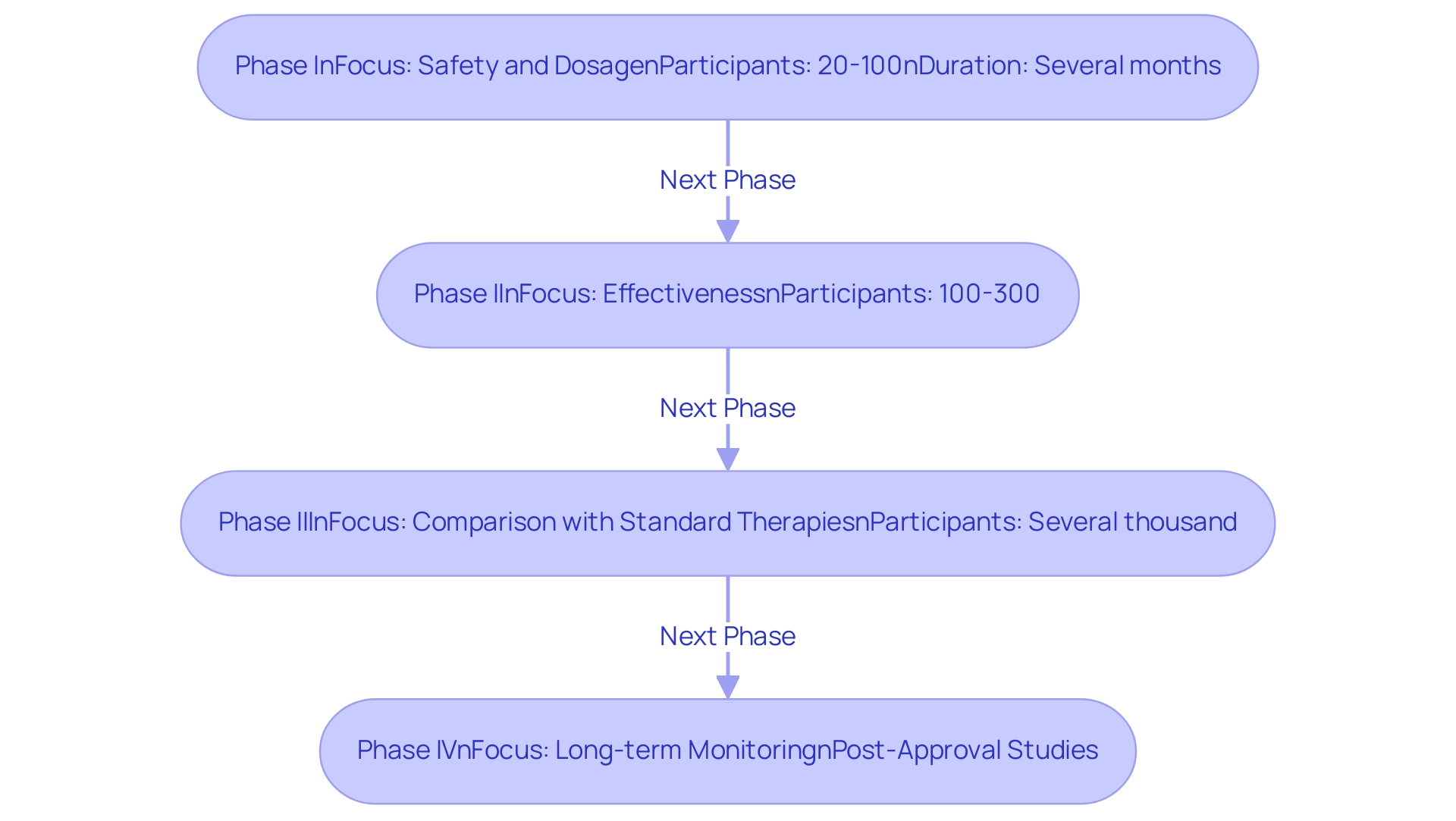
The Dominican Republic Clinical Trials advance through four unique phases, each designed to meet specific goals and needs essential for the creation of safe and effective treatments, especially concerning medical devices in Latin America, where knowledge and regulatory adherence are critical.
-
Phase I: This initial phase primarily focuses on safety and dosage, typically involving a small group of healthy volunteers, often numbering between 20 to 100 participants.
It assesses how the body metabolizes the treatment and identifies any potential side effects. The average duration for Phase I trials can vary, but it generally lasts several months. Notably, the log-likelihood for Biologicals in Model 1 is recorded at -30.453, highlighting the statistical considerations in these early stages.
In areas such as Colombia, managing these requirements is aided by approved clinical research organizations (CROs) like bioaccess®, which focus on Dominican Republic Clinical Trials, in addition to early-feasibility assessments (EFS) and First-In-Human (FIH) trials, while ensuring adherence to local regulations.
-
Phase II: Following successful completion of Phase I, the study expands to a larger cohort, usually comprising 100 to 300 participants.
This phase evaluates the treatment's effectiveness while continuing to monitor safety. The completion rate for Phase II studies is critical, as findings can significantly influence whether a treatment advances to later stages. Logistic regression is frequently used to examine factors influencing study success during this phase, offering valuable insights into treatment effectiveness.
bioaccess® offers comprehensive project management and monitoring services to streamline this process, including Pilot Studies.
-
Phase III: This phase is pivotal, involving several thousand participants across diverse populations to compare the new treatment against standard therapies or placebo controls.
The focus is on confirming the treatment's effectiveness and monitoring side effects, with results that often dictate approval decisions. Recent updates suggest that Phase III evaluations are increasingly emphasized, with calls for more funding to support larger randomized studies that can provide robust data for clinical decision-making. In the context of Dominican Republic Clinical Trials, the ability to adeptly manage study setup, including ethics committee approvals and import permits, is essential in this phase, a service bioaccess® excels in, particularly in managing Pivotal Studies.
-
Phase IV: Conducted post-approval, Phase IV studies are essential for ongoing research that monitors long-term effects and effectiveness in the broader population. These studies assist in collecting real-world evidence and further inform medical practices.
Regulatory bodies such as INVIMA play a critical role in supervising Dominican Republic clinical trials, ensuring these studies adhere to the established guidelines for medical device oversight. Comprehending these phases is essential not only for researchers but also for participants, as it readies them for their involvement and emphasizes the stringent testing treatments undergo before reaching the market. As highlighted by research authority Matej Mikulic, > Contact us now < to explore how bioaccess® can assist with your study requirements and maneuver through these stages efficiently.
Additionally, a recent study examining data from ClinicalTrials. Gov from 2010 to June 2020 employed logistic regression to identify success factors in research studies, highlighting the significance of meticulous data collection and analysis methodologies, which bioaccess® is well-equipped to deliver.
The Recruitment Process: From Pre-Screening to Participation
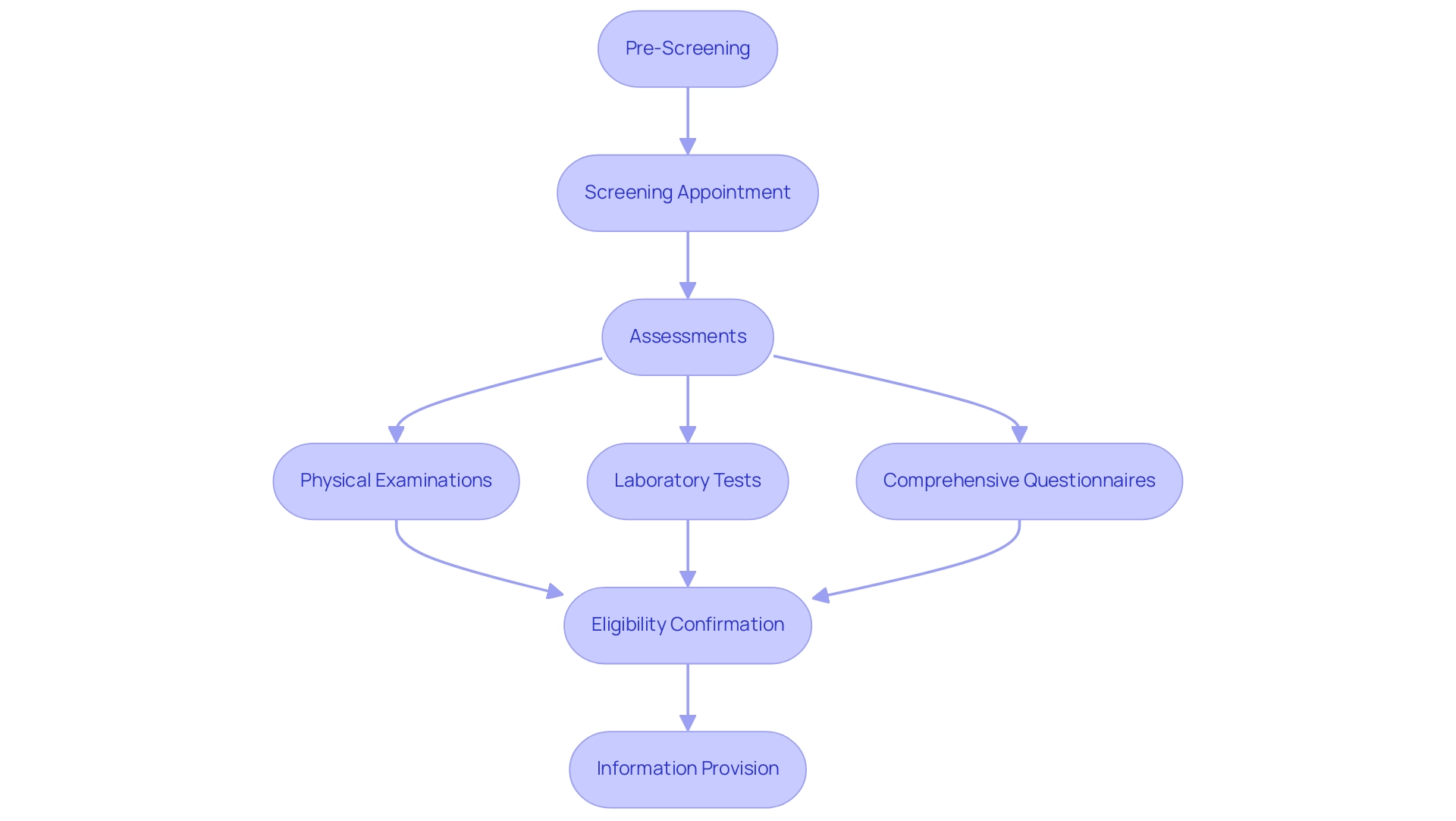
The recruitment process for clinical studies begins with pre-screening, where potential candidates provide essential information about their health history and current medications. This step is crucial, as it allows researchers to identify individuals who may benefit from participation. Following pre-screening, screening appointments are conducted to evaluate eligibility against the specific criteria established in the protocol.
During these visits, a series of assessments may take place, including:
- Physical examinations
- Laboratory tests
- Comprehensive questionnaires
If an individual fulfills the eligibility criteria, they will receive comprehensive details about the study, including its aims, schedule, and the type of involvement. Such openness is essential, as it promotes comfort and readiness among potential contributors.
The significance of efficient recruitment strategies is highlighted by findings that only 2% of nurses reported concerns about losing patients to other healthcare providers as a barrier to referrals. This statistic highlights the potential for streamlined communication and engagement in recruitment efforts, reinforcing the need for researchers to actively address these concerns to enhance referral rates. Moreover, advancements in technology, as noted by Mike Hennessy Jr., emphasize the role of innovative online targeting in reaching patients interested in health topics.
By providing research study information in a significant way, researchers can greatly improve recruitment success rates and ensure a more educated group of individuals.
Recent trends in research participant recruitment processes indicate a shift towards utilizing digital platforms and social media to engage potential participants more effectively. An analysis examining data extraction from the European Registry of Clinical Trials indicated that an Excel database was created to assess recruitment rates and features, revealing insights into the challenges encountered and the necessity for enhanced data management in research.
Furthermore, in the context of Colombia's recent partnership between bioaccess® and Caribbean Health Group, which aims to position Barranquilla as a leading destination for medical research in Latin America, the recruitment process is set to benefit from enhanced visibility and support. This initiative, endorsed by Colombia's Minister of Health, empowers local economies through job creation and improved healthcare services, driving global health improvement through international collaboration and innovation in Medtech. Specifically, bioaccess® will offer extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Project management
These services are anticipated to enhance recruitment efforts.
Simulation training has also been demonstrated to enhance informed consent and pharmacokinetic/pharmacodynamic sampling in pediatric studies, further boosting engagement and comprehension. Grasping the recruitment process not only enables research teams but also fosters a positive experience for individuals contemplating medical studies.
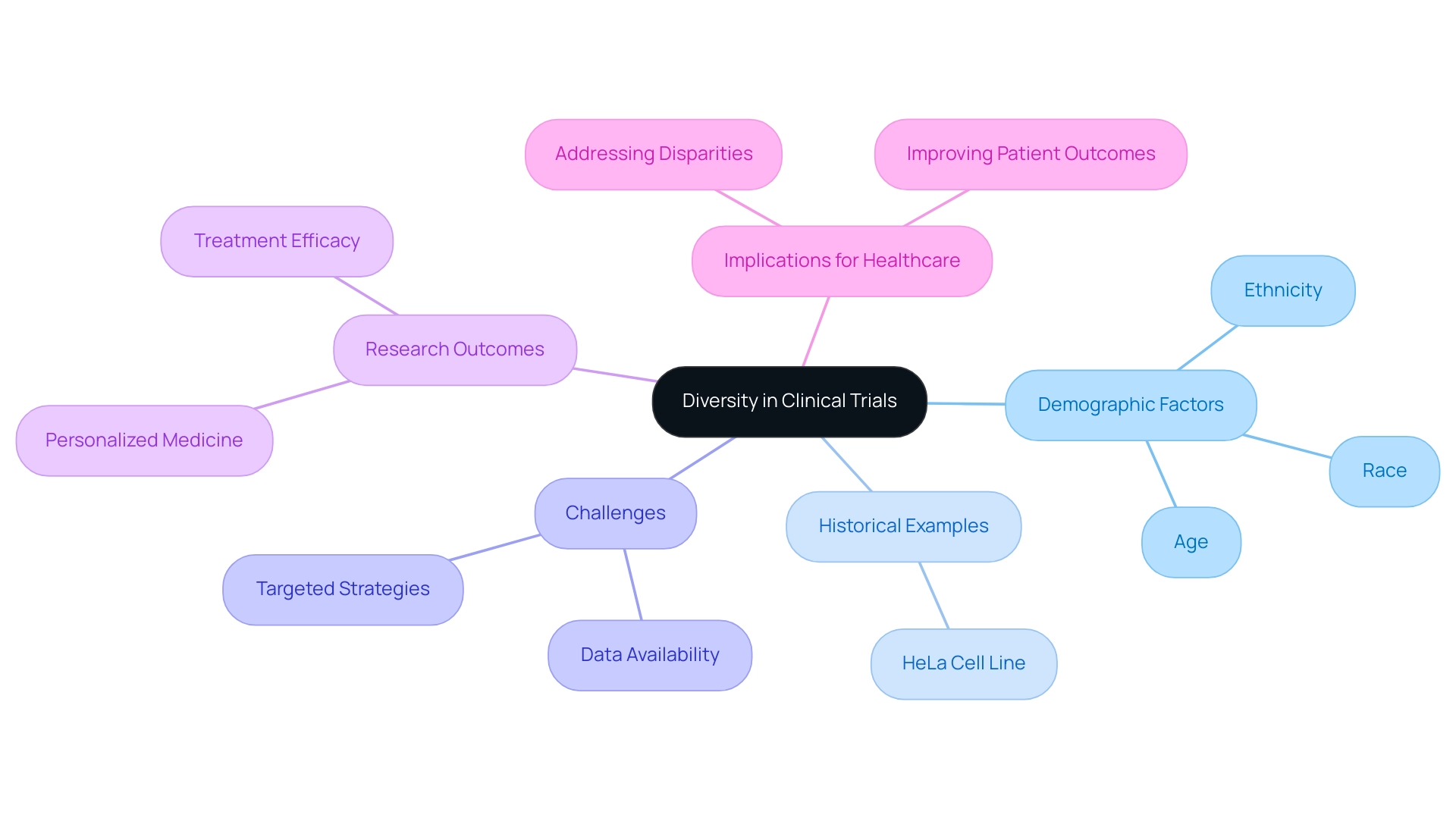
The Importance of Diversity in Clinical Trials: Enhancing Research Outcomes
The inclusion of diverse populations in research trials is essential for ensuring that findings are relevant and beneficial across different demographic groups. By enlisting individuals from diverse backgrounds—including different races, ethnicities, and age groups—clinical trials can offer valuable insights into how treatments perform in a broader context. This diversity not only enhances our understanding of treatment efficacy but also uncovers potential variations in responses and side effects among different groups, which is critical for the advancement of personalized medicine strategies.
A historical instance highlighting the significance of diversity in research is the HeLa cell line, originating from cancerous cervical cells of Henrietta Lacks, which emphasizes the necessity for representative involvement in trials. Moreover, a considerable challenge in enhancing diversity is demonstrated by the case example on the absence of thorough information concerning the racial and ethnic makeup of research participants; without this information, targeted strategies cannot be effectively formulated.
Additionally, recent findings show that asthma disparities are linked to environmental factors, highlighting the necessity for clinicians and researchers to consider where patients live, what they eat, and how they feel—as well as characteristics like race, ethnicity, socioeconomic status, and age—to gain a thorough understanding of their patients’ experiences with asthma symptoms and identify the best preventative strategies or treatment options.
This comprehensive method to patient care highlights the significance of varied involvement in research studies, ensuring that the advantages of medical progress are reachable to all groups in society and promoting more effective healthcare solutions.
Understanding the Role of Ethics Committees in Clinical Trials
Ethics committees, often known as Institutional Review Boards (IRBs), play an essential role in the realm of research studies by reviewing and approving study protocols to ensure compliance with established ethical standards and regulations. Their essential duty is to protect the rights, safety, and welfare of individuals. This is achieved through rigorous evaluation processes that ensure informed consent is obtained and that the research is conducted with ethical integrity.
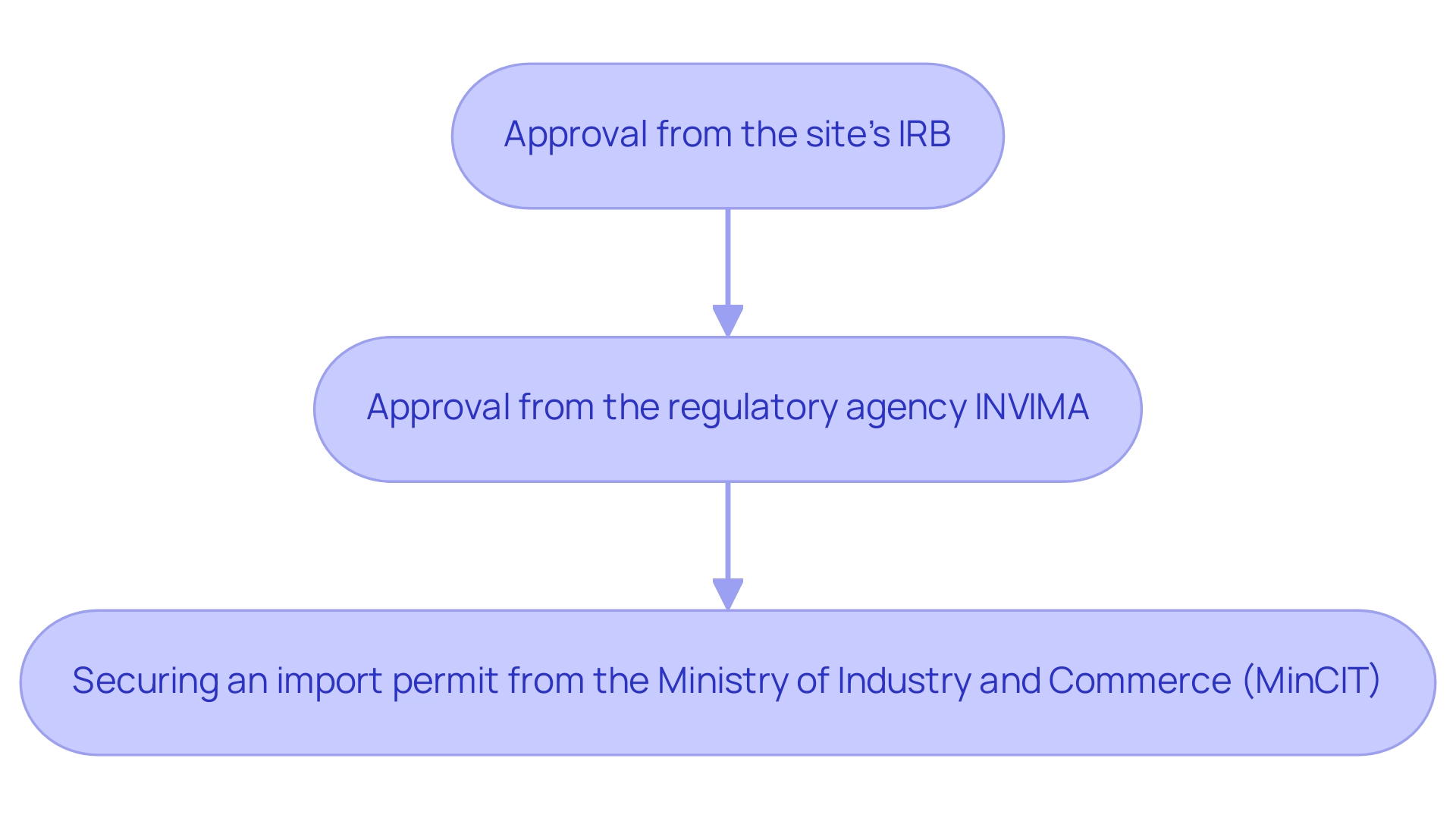
IRBs assess the potential risks and benefits associated with Dominican Republic Clinical Trials, carefully balancing the need for scientific advancement with the safety of participants. In Colombia, obtaining approval for research involves a clear sequential process:
- Approval from the site's IRB
- Approval from the regulatory agency INVIMA
- Securing an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices
Notably, compliance rates with ethical standards remain a pressing concern; recent data indicates that violations of IRB rules account for 4-5% of all retractions in published research, highlighting the critical need for stringent oversight.
Furthermore, challenges arise in reviewing studies involving complementary and integrative medicine (CIM), where a lack of quality evidence and regulatory guidance often forces IRBs to operate within limited frameworks. As highlighted by a member of the ethics committee, 'The function of IRBs is crucial in upholding ethical standards and safeguarding individuals, particularly in regions where evidence is limited.'
Furthermore, medical device startups encounter considerable regulatory challenges, including competition, recruitment issues, and financial limitations, which complicate the trial environment and can postpone the approval process.
Understanding the essential functions of ethics committees empowers participants to recognize the safeguards in place during Dominican Republic Clinical Trials, ensuring their interests are protected throughout the research process.
Conclusion
The exploration of clinical trials reveals their indispensable role in advancing medical science and improving patient care. These meticulously structured studies not only assess the safety and effectiveness of new treatments but also contribute significantly to the body of medical knowledge. With an impressive 94% of interventional studies reporting their findings as of May 2023, the collaborative efforts of researchers and institutions are crucial in enhancing healthcare outcomes.
Understanding the rights of participants is paramount, as these rights ensure ethical treatment and informed consent throughout the research process. Participants must be aware of their ability to withdraw from trials and the importance of clear communication regarding trial procedures. Additionally, the recruitment process plays a vital role in ensuring a diverse and representative participant base, which is essential for generating applicable and meaningful results across varied populations.
The phases of clinical trials—from safety assessments in Phase I to long-term effectiveness evaluations in Phase IV—illustrate the rigorous testing that new treatments undergo before reaching the market. Each phase serves a distinct purpose, ultimately contributing to the overall integrity and reliability of clinical research. Furthermore, the significance of ethics committees cannot be overstated, as they provide necessary oversight to protect participants and uphold ethical standards.
In summary, clinical trials are a cornerstone of medical innovation, driving advancements that benefit all segments of society. By fostering diverse participation, ensuring ethical compliance, and navigating the complexities of the research process, these trials pave the way for improved healthcare outcomes worldwide. The commitment to transparency, participant rights, and ethical standards is essential in this ongoing journey toward medical excellence.
Frequently Asked Questions
What are clinical experiments?
Clinical experiments are meticulously crafted research projects aimed at assessing the effectiveness and safety of new medical interventions, including drugs, therapies, and procedures.
Why are clinical studies important?
Clinical studies are essential for ensuring that new treatments are safe and effective, ultimately leading to improved patient outcomes and advancing medical knowledge.
What percentage of interventional research studies have posted their results as of May 2023?
As of May 2023, 94% of interventional research studies have posted their results.
What challenges do Medtech companies in Latin America face?
Medtech companies in Latin America face significant challenges such as regulatory hurdles, limited financial resources, and a fragmented healthcare system that complicates collaboration.
What is the partnership between bioaccess® and Caribbean Health Group aiming to achieve?
The partnership aims to position Barranquilla as a leading destination for medical studies in Latin America, supported by Colombia's Minister of Health.
What recent trends are impacting the landscape of clinical studies?
Recent trends include the acquisition of a contract research organization (CRO) by a pharmaceutical company in September 2023 and the merger of two research firms in December 2023.
Can participants in clinical studies withdraw at any time?
Yes, participants have the right to withdraw from the study at any time without facing penalties.
What is the right to informed consent in clinical studies?
The right to informed consent mandates that individuals receive comprehensive information regarding the study’s purpose, procedures, potential risks, and benefits before agreeing to participate.
What percentage of individuals understand the concept of randomization in clinical studies?
Current statistics reveal that only 39.4% of individuals understand the concept of randomization.
Why is patient empowerment significant in clinical studies?
Patient empowerment is significant as it enhances transparency and engagement in the research process, especially as informed consent procedures continue to develop.
What are the rights of participants in clinical studies?
Participants possess rights that safeguard their well-being, including the right to informed consent, the right to withdraw from the study, the right to seek answers to questions, and the right to privacy and confidentiality regarding their personal data.

