Overview
The article examines the evolving landscape of medical research trends in Latin America, emphasizing significant advancements in clinical trials, regulatory innovations, and the pivotal role of Contract Research Organizations (CROs) in bolstering research capabilities. It highlights that, despite challenges such as socioeconomic disparities and regulatory complexities, the region is strategically positioned for growth. This potential is fueled by increasing investments, technological advancements, and a concentrated focus on personalized medicine, which collectively enhance Latin America's participation in global clinical trials.
Introduction
The medical research landscape in Latin America stands on the cusp of a transformative era, propelled by innovative clinical trials and a steadfast commitment to enhancing healthcare outcomes. With countries such as Brazil, Mexico, and Argentina at the forefront, this region is leveraging its diverse demographics and evolving infrastructure to tackle urgent health issues. Landmark studies, including the Hispanic Community Health Study, underscore the significance of understanding health disparities, while collaborations aimed at establishing cities like Barranquilla as clinical trial hubs indicate a burgeoning investment in research.
Nevertheless, challenges persist, including regulatory obstacles and funding disparities, which necessitate strategic partnerships and innovative solutions. As the region embraces decentralized clinical trials and regulatory innovations, the potential for substantial advancements in medical research becomes increasingly evident, heralding a future abundant with opportunities to address both local and global health challenges.
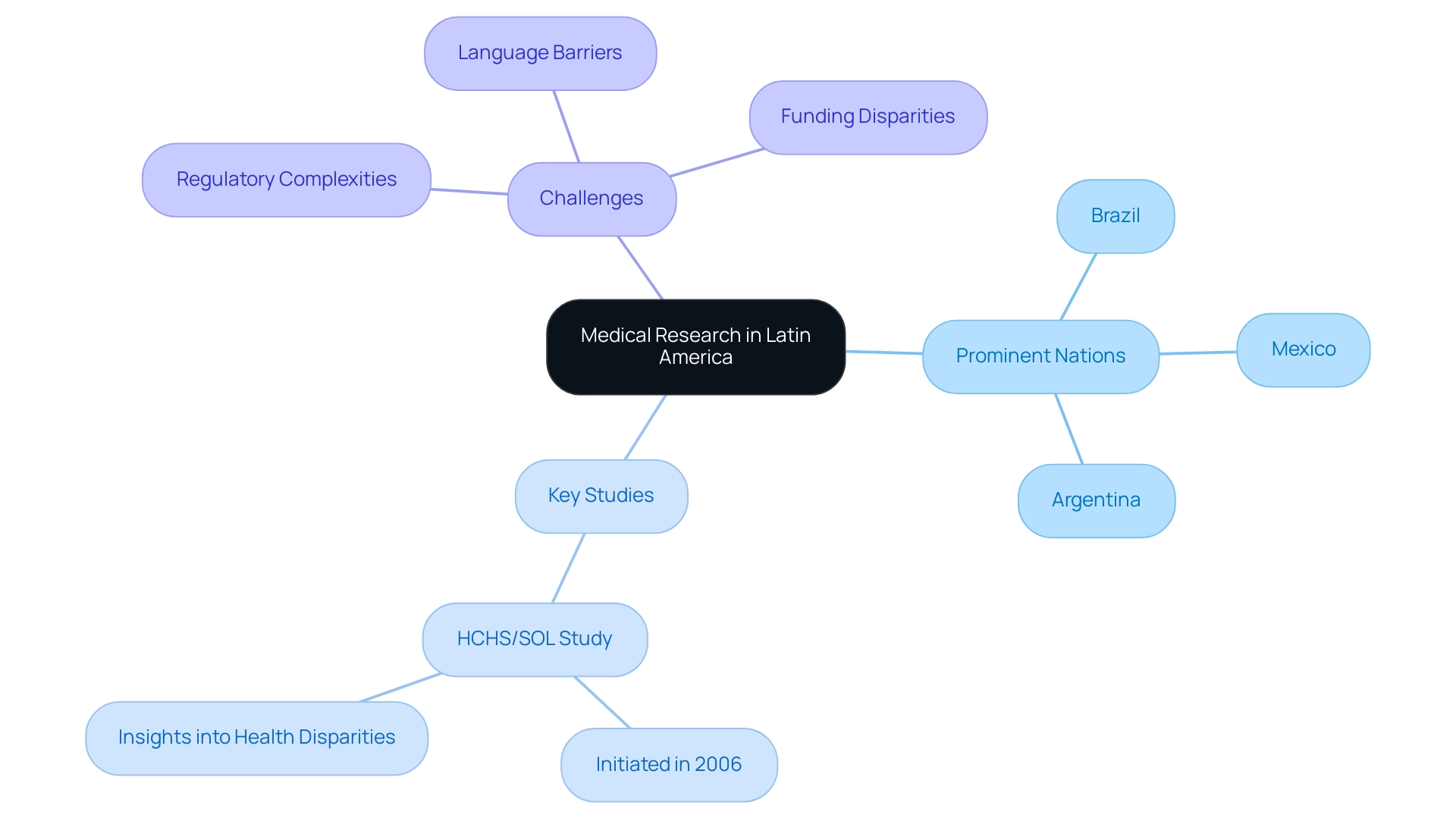
Current Landscape of Medical Research in Latin America
The medical research trends in Latin America are undergoing significant transformation, marked by an increasing focus on enhancing healthcare outcomes through robust clinical trials and studies. Prominent nations like Brazil, Mexico, and Argentina benefit from a varied patient demographic and a developing infrastructure that supports innovative methods. A notable example of ongoing investigative efforts in the region is the landmark Hispanic Community Health Study/Study of Latinos (HCHS/SOL), initiated in 2006, which has provided valuable insights into health disparities and outcomes among Hispanic populations.
Furthermore, partnerships between entities such as bioaccess™ and Caribbean Health Group aim to establish Barranquilla as a premier location for research in Latin America, with the assistance of Colombia's Minister of Health. Despite these advancements, the region faces persistent challenges, including regulatory complexities, language barriers, and disparities in funding, which hinder effective communication and collaboration. Efforts to overcome these obstacles are supported by increasing partnerships with global study organizations, promoting knowledge sharing and enhancing resources—crucial for advancing medical research trends in Latin America, especially in addressing urgent local health challenges such as non-communicable diseases, infectious diseases, and cancer.
As highlighted by Jennifer Mendoza, an expert in health and pharmaceuticals, 'Due to varying update cycles, statistics can display more current data than mentioned in the text,' emphasizing the need for ongoing observation of clinical studies. This is particularly relevant in Brazil, Mexico, and Argentina, where ongoing studies are shaping the future of healthcare in the region. The incorporation of data visualization tools, like those offered by the ICTRP, enables stakeholders to examine registration trends dynamically, demonstrating how these developments can enhance decision-making in studies.
However, it is essential to address data gaps to ensure uniform classification and reliable insights into the medical funding landscape in Latin America for 2024.
Emerging Trends in Clinical Research and Regulatory Innovations
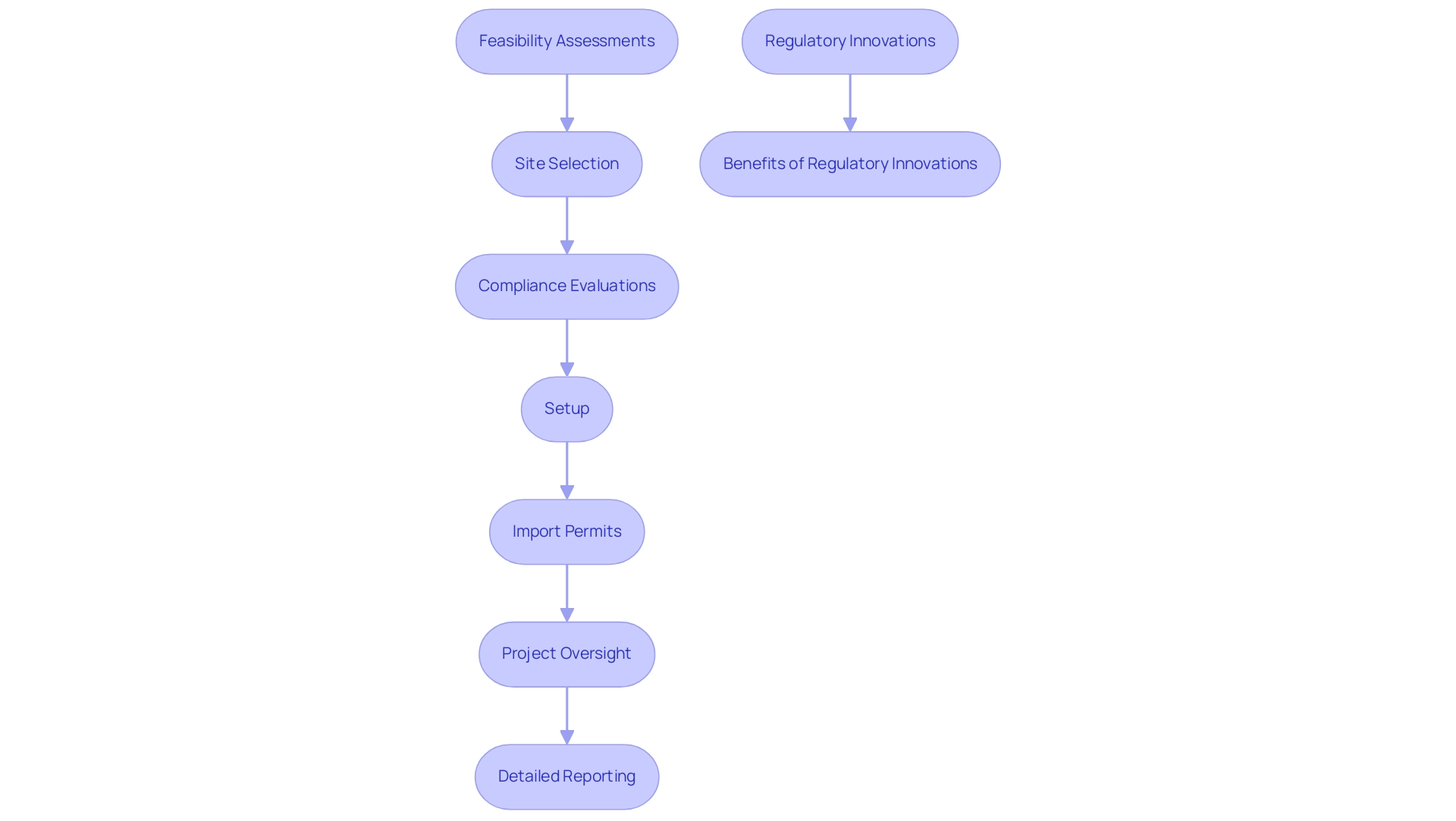
The research environment in Latin America is undergoing transformative trends, particularly in medical research, marked by the increasing implementation of decentralized studies (DCTs). These studies leverage advanced technology to enhance patient recruitment and retention while minimizing data transcription errors, thus significantly improving data accuracy. bioaccess® is at the forefront of this evolution, providing extensive management services for research, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Detailed reporting that encompasses the monitoring of serious and non-serious adverse events
Notably, Syneos Health has launched a DCT site network to promote the uptake of DCTs, offering high-quality solutions that facilitate this shift. Regulatory innovations, such as expedited approval processes and the harmonization of regulations across countries, are gaining momentum. These initiatives encourage streamlined ethics committee reviews and enhance regulatory compliance, fostering a more efficient trial environment. Such advancements not only attract more researchers to engage in medical research that directly benefits local populations but also contribute to local economies through job creation and healthcare improvement.
For instance, DCTs utilize digital tools to collect and analyze large amounts of data from diverse cultural and ethnic backgrounds, significantly aiding in the understanding of new therapeutics. The incorporation of real-world evidence into regulatory submissions is transforming the assessment of outcomes, ensuring that the insights gained reflect broader populations. With approximately 1.9 million new cancer instances anticipated in the United States for 2022, the significance of effective studies and varied data gathering becomes even more pronounced.
As Laura Wood from ResearchAndMarkets.com observes, ResearchAndMarkets.com is the world's foremost provider of [international market research reports](https://globenewswire.com/news-release/2025/02/25/3031708/0/en/Decentralized-Clinical-Trials-DCTs-Research-Report-2025-Transforming-Drug-Development-with-Remote-Monitoring-Digital-Tools-Global-Market-Trends-Opportunities-and-Forecasts-2020-203.html) and market data, emphasizing the importance of these innovations in enhancing the standards of medical research in Latin America. Furthermore, adherence to country requirements is essential to guarantee that all research documents are reviewed and approved, and that import permits and the nationalization of investigational devices are effectively managed as part of the setup process.
Challenges in Conducting Clinical Research: Recruitment and Compliance
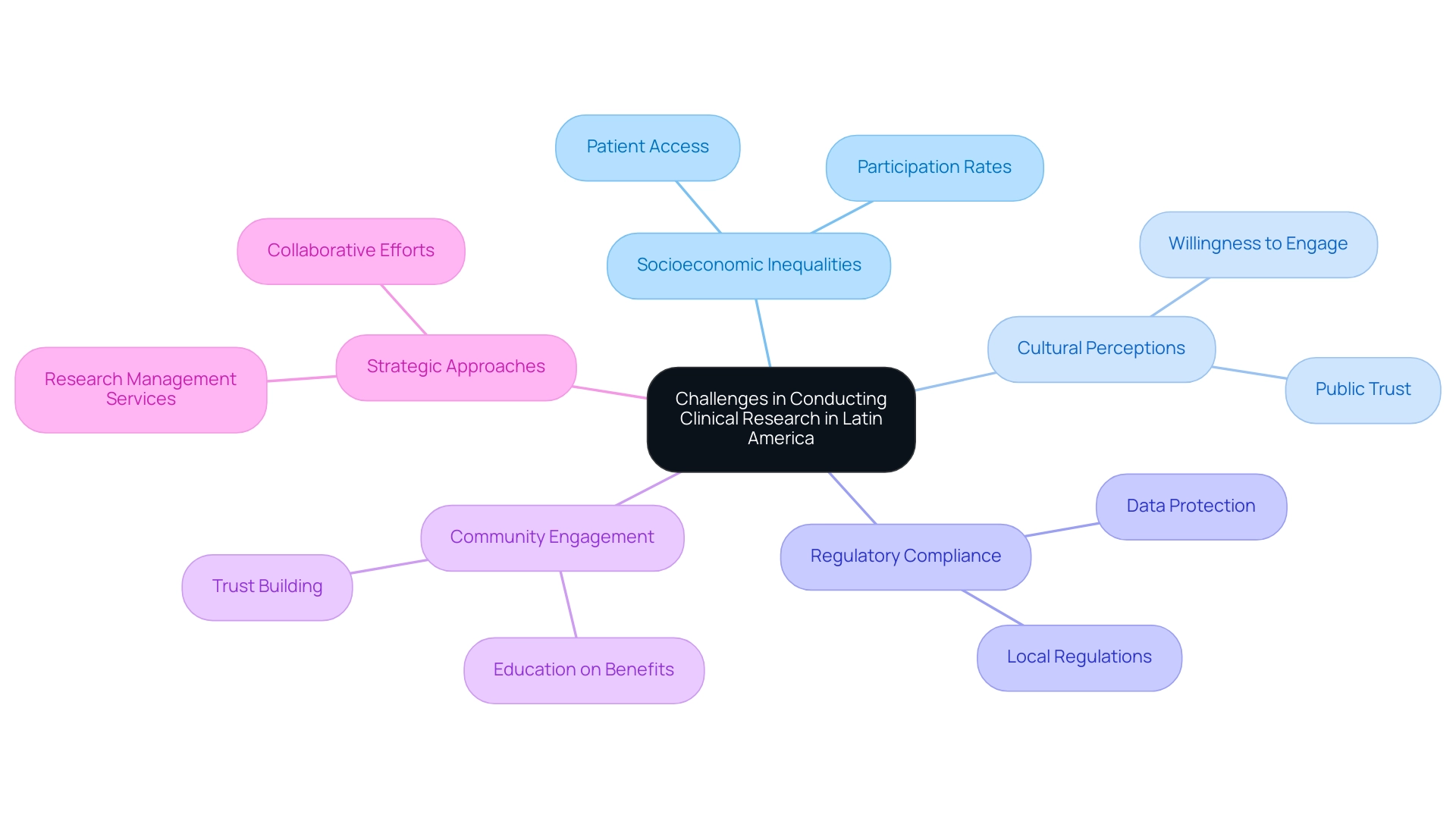
Recruitment for research trials in Latin America presents significant challenges that can hinder progress in medical research trends within the region. Socioeconomic inequalities restrict patient access and participation rates, while cultural perceptions of clinical research influence public trust and willingness to engage in trials. Additionally, adherence to various local regulations complicates timelines and execution.
As noted by Gotuzzo from Universidad Peruana Cayetano Heredia, these challenges demand a strategic approach to recruitment. Notably, Colombia has achieved over a 50% reduction in recruitment time and boasts 95% retention rates, demonstrating the effectiveness of community-based recruitment strategies. Engaging local stakeholders not only fosters trust but also educates communities about the benefits of participation, which is essential in this context.
Insights from the case analysis 'Future Perspectives on Clinical Trials in Latin America' underscore the necessity for enhanced infrastructure and collaboration among stakeholders to optimize resources, aligning with the medical research trends in the region. Furthermore, addressing data protection and grievance handling, as outlined in our FAQs, is vital for building trust and ensuring compliance in medical studies. By collaborating with local organizations and leveraging bioaccess®’s extensive research management services, researchers can navigate the regulatory landscape more effectively, ensuring compliance and optimal execution of their projects.
These concerted efforts not only improve recruitment rates but also contribute to the overall success of clinical trials, reflecting the prevailing medical research trends in Latin America. Testimonials from prior research highlight the effectiveness of these strategies, illustrating tangible success in enhancing participation and trust.
The Role of CROs in Advancing Medical Research
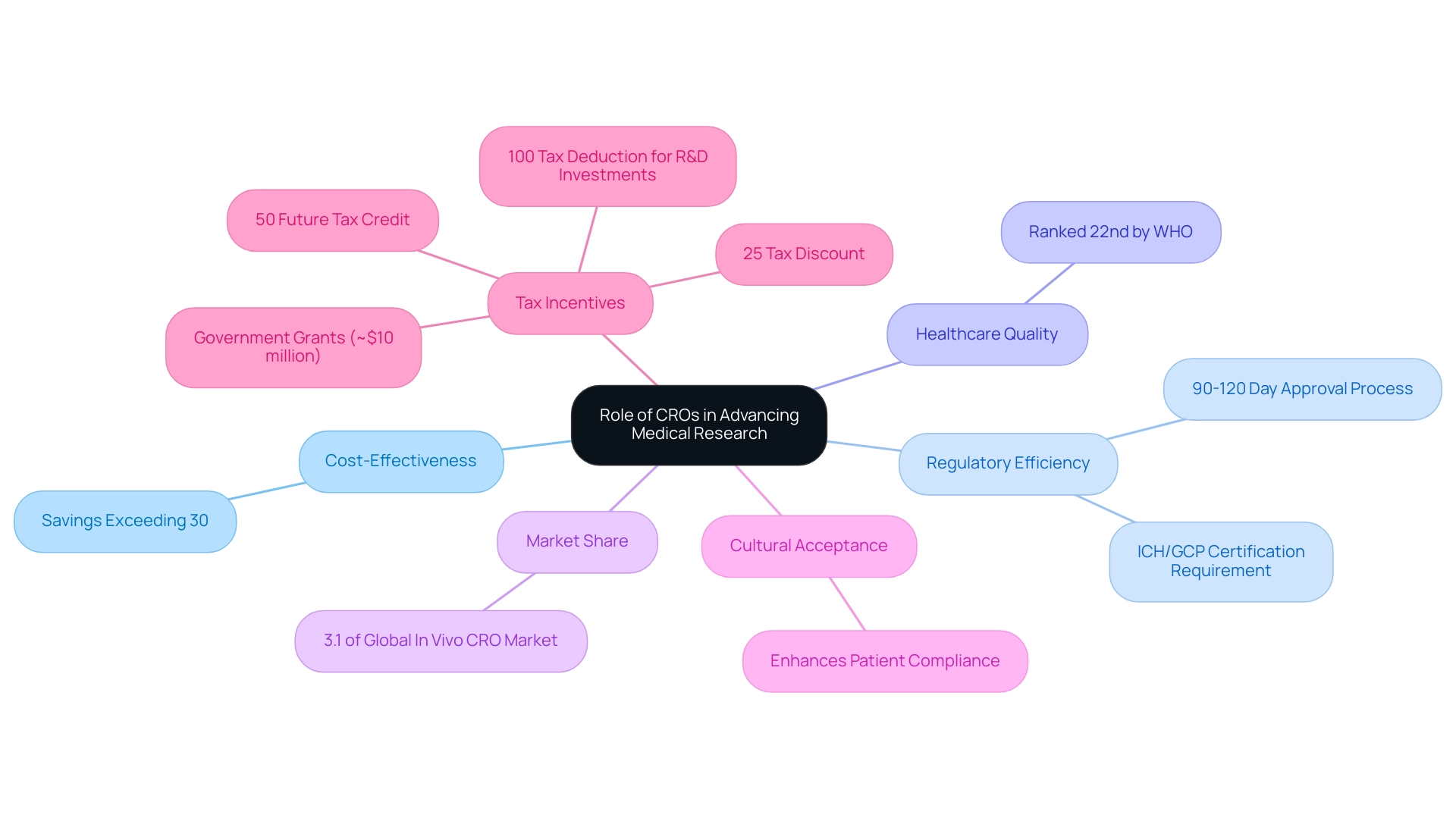
Contract Research Organizations (CROs) are pivotal in shaping medical research trends in Latin America, with Colombia standing out due to its cost-effectiveness, regulatory efficiency, and high-quality healthcare. Savings exceeding 30% compared to trials in North America or Western Europe, coupled with a streamlined IRB/EC and INVIMA approval process that typically takes only 90-120 days, position Colombia as a prime destination for first-in-human studies. The country boasts a robust healthcare system, ranked 22nd by the World Health Organization, where hospitals are required to achieve stringent ICH/GCP certification for trials.
In 2023, Latin America represented 3.1% of the global in vivo CRO market, highlighting the growing significance of medical research trends in the region. CROss like bioaccess® exemplify operational excellence in this landscape, offering comprehensive services from design to patient recruitment. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, noted that his experience with bioaccess® during the initial human study in Colombia illustrated the effectiveness of local CROs in navigating the regulatory framework.
Moreover, cultural acceptance of medical study participation significantly enhances patient compliance, which is essential for the success of medical research trends in Latin America. The oncology segment is particularly noteworthy, thriving on the expansion of precision medicine and the rising demand for specialized CROs skilled in managing complex regulatory requirements influenced by these trends. This is further evidenced by strategic moves within the CRO market, such as Precision for Medicine's acquisition of Baseline Controls, underscoring the sector's commitment to enhancing research capabilities and patient access to innovative therapies.
Additionally, Colombia offers substantial R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants, further enhancing its appeal as a trial destination. Understanding the IRB/EC and INVIMA approval processes is crucial, as these regulatory pathways ensure that investigations are conducted ethically and efficiently, reinforcing Colombia's status as a leader in medical trials.
Future Outlook: Opportunities and Growth in Medical Research
The outlook for medical research trends in Latin America is exceptionally encouraging, with projections indicating that medicine use in the region will grow faster than in other areas over the next five years. This expansion is primarily fueled by escalating investments in healthcare, technological advancements, and an increasing emphasis on personalized medicine. Comprehensive research study management services—including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting—are pivotal in driving this growth.
Specifically, the setup process involves obtaining necessary approvals from ethics committees and health ministries, which ensures adherence to country regulations and enhances the reliability of findings. Notably, significant contributors to this expansion include oncology, immunology, diabetes, and obesity drugs, which are expected to drive demand and innovation. As nations within the region improve their investigative infrastructures and streamline regulatory processes, the medical research trends in Latin America indicate it is poised to become a significant participant in global clinical trials.
Additionally, burgeoning fields such as gene therapy and digital health interventions offer distinct avenues for innovation and development. The increasing emphasis on health equity and enhanced access to care is anticipated to motivate a surge in initiatives aimed at addressing local and regional health challenges. As Roshan Deshmukh states, 'The future of medical exploration in Latin America is bright, with unique opportunities for innovation that can address both local and global health needs.'
Collectively, these factors, along with the impact of Medtech clinical studies on local economies—such as job creation, economic growth, and international collaboration—highlight the medical research trends in Latin America, positioning the region as an evolving hub for clinical research that capitalizes on its diverse populations and unique health needs.
Conclusion
The medical research landscape in Latin America is on the brink of extraordinary growth, propelled by innovative clinical trials and a steadfast commitment to enhancing healthcare outcomes. Countries such as Brazil, Mexico, and Argentina are harnessing their diverse demographics and advancing research infrastructure to confront substantial health challenges. Landmark studies, including the Hispanic Community Health Study, underscore the necessity of understanding health disparities. Additionally, initiatives to establish cities like Barranquilla as clinical trial hubs reflect a robust investment in research initiatives.
Despite these promising advancements, challenges persist, including regulatory hurdles and funding disparities. It is essential to address these issues through strategic partnerships and innovative solutions. The emergence of decentralized clinical trials and regulatory innovations signals a pivotal shift in the region, presenting new opportunities to boost patient engagement and enhance data accuracy. As the region continues to embrace these transformations, the potential for significant advancements in medical research becomes increasingly evident.
Moreover, the role of Contract Research Organizations (CROs) is crucial in advancing the industry, especially in Colombia, where cost efficiency and regulatory speed render it an appealing destination for clinical studies. The future outlook for medical research in Latin America is optimistic, with anticipated growth in medicine usage and a focus on personalized healthcare solutions. As the region fortifies its research capabilities and prioritizes health equity, it is well-positioned to emerge as a significant player in the global clinical research arena, ultimately benefiting both local and international health initiatives.
Frequently Asked Questions
What are the current trends in medical research in Latin America?
Medical research in Latin America is experiencing significant transformation, with a focus on enhancing healthcare outcomes through robust clinical trials and decentralized studies (DCTs). Countries like Brazil, Mexico, and Argentina are leading these efforts, leveraging advanced technology for patient recruitment and data accuracy.
What is the Hispanic Community Health Study/Study of Latinos (HCHS/SOL)?
The HCHS/SOL, initiated in 2006, is a landmark study aimed at providing insights into health disparities and outcomes among Hispanic populations, contributing valuable data to the understanding of health issues in these communities.
What challenges does medical research in Latin America face?
The region faces challenges such as regulatory complexities, language barriers, and disparities in funding, which hinder effective communication and collaboration in medical research.
How are partnerships contributing to medical research in Latin America?
Partnerships, such as those between bioaccess™ and Caribbean Health Group, aim to establish locations like Barranquilla as premier research hubs. These collaborations promote knowledge sharing and enhance resources, crucial for addressing urgent health challenges.
What role do decentralized clinical trials (DCTs) play in Latin American research?
DCTs utilize advanced technology to improve patient recruitment and retention while minimizing data transcription errors, thus enhancing data accuracy. They enable the collection and analysis of large amounts of data from diverse populations, which aids in understanding new therapeutics.
What innovations are being introduced to streamline medical research in Latin America?
Innovations such as expedited approval processes and the harmonization of regulations across countries are being implemented to streamline ethics committee reviews and enhance regulatory compliance, fostering a more efficient trial environment.
How do DCTs impact local economies and healthcare?
DCTs attract more researchers to engage in medical research that benefits local populations and contribute to local economies through job creation and healthcare improvements.
Why is addressing data gaps important in medical research funding?
Addressing data gaps is essential for ensuring uniform classification and reliable insights into the medical funding landscape in Latin America, which is crucial for effective decision-making and resource allocation for future studies.




