Introduction
The General Safety and Performance Requirements (GSPR) stand as a pivotal framework within the realm of medical device regulation, emphasizing the critical balance between ensuring patient safety and fostering innovation in the healthcare sector.
As manufacturers strive to meet these stringent criteria set forth by the Medical Device Regulation (MDR), a comprehensive understanding of GSPR becomes essential.
This article delves into the multifaceted aspects of GSPR compliance, exploring the systematic processes required, the challenges faced by manufacturers, and the best practices that can enhance adherence to these vital standards.
By examining the role of documentation, risk management, and post-market surveillance, this discussion aims to equip stakeholders with the knowledge necessary to navigate the complexities of regulatory compliance in an ever-evolving landscape.
Understanding General Safety and Performance Requirements (GSPR)
GSPR medical devices are essential standards mandated by the Medical Product Regulation (MDR) that healthcare products must fulfill to ensure their safety and efficacy. These requirements are pivotal in safeguarding public health while fostering innovation within the healthcare technology sector. GSPR encompasses various benchmarks, including:
- Risk management
- Clinical evaluation
- Comprehensive post-market surveillance
Notably, manufacturers are required to make the results of postmarket equipment studies publicly available in a timely manner, enhancing transparency and accountability in performance. As emphasized by Pontus Gedda, a medical equipment expert, understanding GSPR is essential for manufacturers to navigate the intricacies of adherence and ensure their market access. In this context, it is essential to note that manufacturers choose and compensate one of approximately 80 for-profit, private Notified Bodies to assess their product and obtain a CE mark, which is vital for understanding the regulatory landscape.
Furthermore, the FDA's stringent postmarket surveillance activities, including adverse event reporting and ongoing assessments, demonstrate the agency's commitment to monitoring safety once they are on the market. For instance, the FDA mandates various surveillance activities to safeguard public health, which are outlined in case studies on postmarket surveillance requirements. With the latest updates on MDR adherence set for 2024, including new guidance on agency review processes and the requirement for substantive summaries of significant decisions regarding apparatus applications, the impact of GSPR medical devices on healthcare product market access is becoming increasingly significant.
This requires that manufacturers not only fulfill these essential criteria but also actively interact with new compliance frameworks, such as those managed by INVIMA in Colombia, which plays a vital role in health product supervision and categorization as a Level 4 health authority by PAHO/WHO. Utilizing the knowledge of teams such as bioaccess®, which focuses on clinical trial management services—including feasibility studies, site selection, regulatory reviews, trial setup, import permits, and project management—can greatly improve manufacturers' capacity to navigate these complexities under the direction of specialists like Katherine Ruiz, who provide essential insights into regulatory matters for healthcare products and in vitro diagnostics.
Step-by-Step Process to Achieve GSPR Compliance
Achieving General Safety and Performance Requirements (GSPR) compliance involves a systematic approach that encompasses several critical steps:
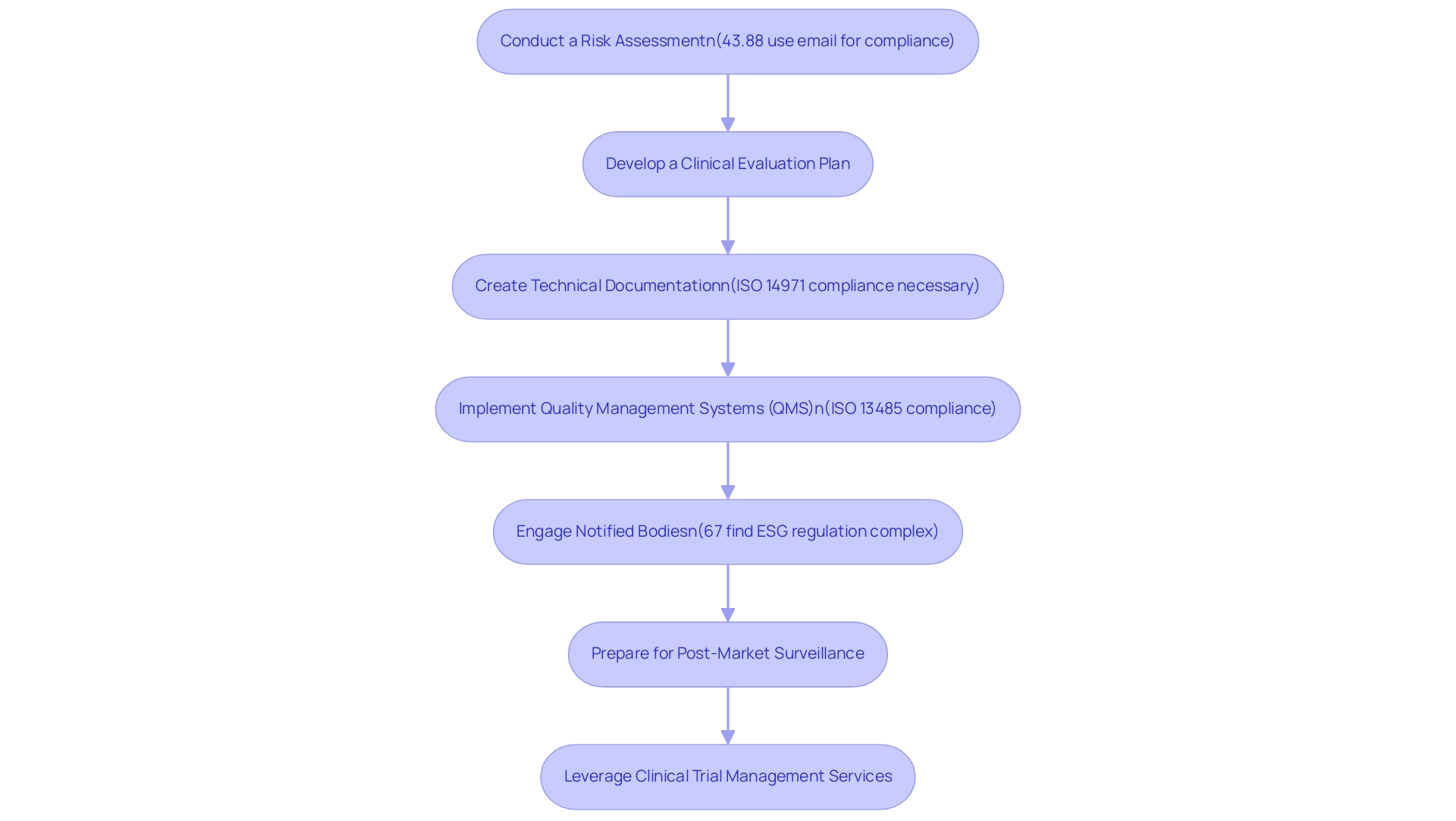
- Conduct a Risk Assessment: Begin by identifying potential hazards associated with your medical equipment. Evaluate the associated risks to ensure that all possible safety concerns are addressed. Recent studies indicate that a substantial 43.88% of healthcare facilities utilize email for compliance communications, highlighting the importance of effective documentation and communication in risk assessments.
- Develop a Clinical Evaluation Plan: Formulate a comprehensive strategy for collecting clinical data that substantiates the safety and performance of your product. This plan should clearly outline how clinical data will be gathered, analyzed, and utilized to meet compliance expectations.
- Create Technical Documentation: Compile a thorough technical file that includes essential documentation such as design specifications, manufacturing processes, and a complete risk management file. Manufacturers must establish and maintain this risk management file, which compiles all documents created during the risk management process. This documentation acts as a basis for oversight assessment and must demonstrate conformity with ISO 14971 standards, as adherence is vital for market entry in significant healthcare markets, including the US and EU. A case study titled "Regulatory Requirements on Medical Device Risk Management" highlights that compliance with ISO 14971 is necessary to meet official expectations and ensure a solid risk management system.
- Implement Quality Management Systems (QMS): Ensure that your organization is compliant with ISO 13485 standards, which outline the requirements for a quality management system specifically for medical product manufacturing. A strong QMS is vital for ensuring product quality and complying with standards.
- Engage Notified Bodies: Collaborate with a Notified Body for conformity assessment. These organizations play a crucial role in verifying that your equipment meets all regulatory requirements, thus facilitating its entry into the market. This step is particularly important as 67% of executives perceive ESG regulations as overly complex, with 70% seeking more guidance from regulators. As Beazley observes, "67% of global executives think that ESG regulation is overly complicated, while 70% desire more direction from regulators," emphasizing the need for clarity in adherence pathways.
- Prepare for Post-Market Surveillance: Develop a proactive plan for ongoing monitoring of the device’s safety and performance once it is on the market. This monitoring is essential for ensuring that any potential risks are identified and managed promptly, thus maintaining adherence post-launch.
- Leverage Clinical Trial Management Services: Utilize comprehensive clinical trial management services, including feasibility studies, site selection, trial setup, import permits, project management, and review processes, to streamline the GSPR adherence process. These services offer vital assistance in navigating compliance requirements and enhancing the efficiency of clinical trials.
By adhering to these steps and incorporating clinical trial management services, organizations can improve their likelihood of attaining GSPR adherence efficiently for GSPR medical devices, ensuring that their healthcare products meet vital safety and performance criteria while maneuvering through the intricacies of legal obligations. This approach aligns with INVIMA's oversight in Colombia, ensuring a thorough understanding of the local compliance landscape.
Navigating Challenges in GSPR Compliance
Manufacturers encounter numerous challenges in achieving compliance with GSPR medical devices, which include:
-
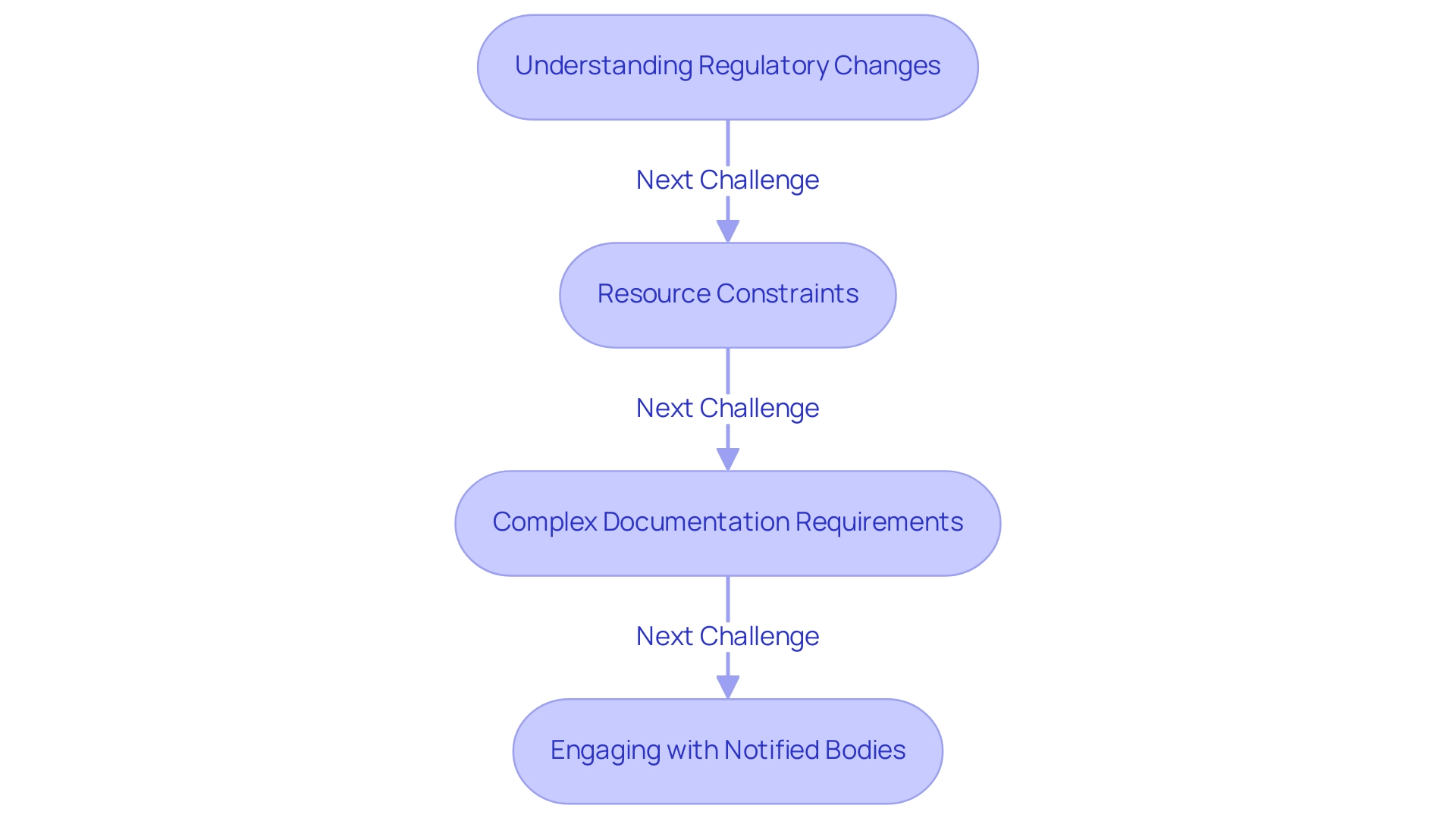
Understanding Regulatory Changes: The landscape of medical device regulations is continually evolving, making it imperative for manufacturers to stay informed through ongoing education and industry updates. The recent transition to the EU Medical Device Regulation (EU MDR) illustrates the complexities involved, as it replaced the old Medical Device Directive (MDD) on May 26, 2021, broadening the scope of products and introducing new definitions and classifications. As noted, many manufacturers must change their approach or risk having their products' approvals revoked after the 2021 deadline.
This shift underscores the need for manufacturers to align their strategies with the dual goals of ensuring patient safety while fostering innovation in product development related to GSPR medical devices.
-
Resource Constraints: Compliance efforts can be severely impacted by limited budgets and personnel. Manufacturers are encouraged to prioritize resources effectively and consider engaging external expertise, such as that offered by regulatory consultants like Ana Criado, who has extensive experience in this field. Ana can offer customized strategies for resource management, assisting manufacturers in optimizing their available assets and expertise to effectively achieve adherence to GSPR medical devices.
-
Complex Documentation Requirements: The extensive documentation required for meeting standards can be overwhelming for many organizations. The analogue GSPR checklist for IVDR 2017/746, an 80-page document, exemplifies the depth of documentation needed for GSPR medical devices, highlighting the complexity and thoroughness necessary for adherence. To streamline this process, manufacturers should develop a structured documentation strategy that ensures all necessary information is captured efficiently. Ana's knowledge in health economics can help in pinpointing crucial documentation priorities that align with official expectations.
-
Engaging with Notified Bodies: Effective communication with Notified Bodies is essential for successful adherence. Manufacturers must prepare thoroughly for audits, maintaining transparency throughout the process to foster a collaborative relationship. This proactive strategy can greatly improve adherence results and enable more seamless interactions with authorities. Moreover, considering the enhanced oversight structure set by the EU MDR, producers may implement particular adherence strategies such as thorough risk evaluations and ongoing monitoring to guarantee alignment with GSPR medical devices and the new standards. Experts such as Ana Criado, with her experience in oversight matters and biomedical engineering, can offer valuable insights and strategies to navigate these complexities, including case studies of successful adherence initiatives she has led.
Best Practices for Ensuring GSPR Compliance
To achieve and maintain compliance with General Safety and Performance Requirements (GSPR), organizations should adopt the following best practices:
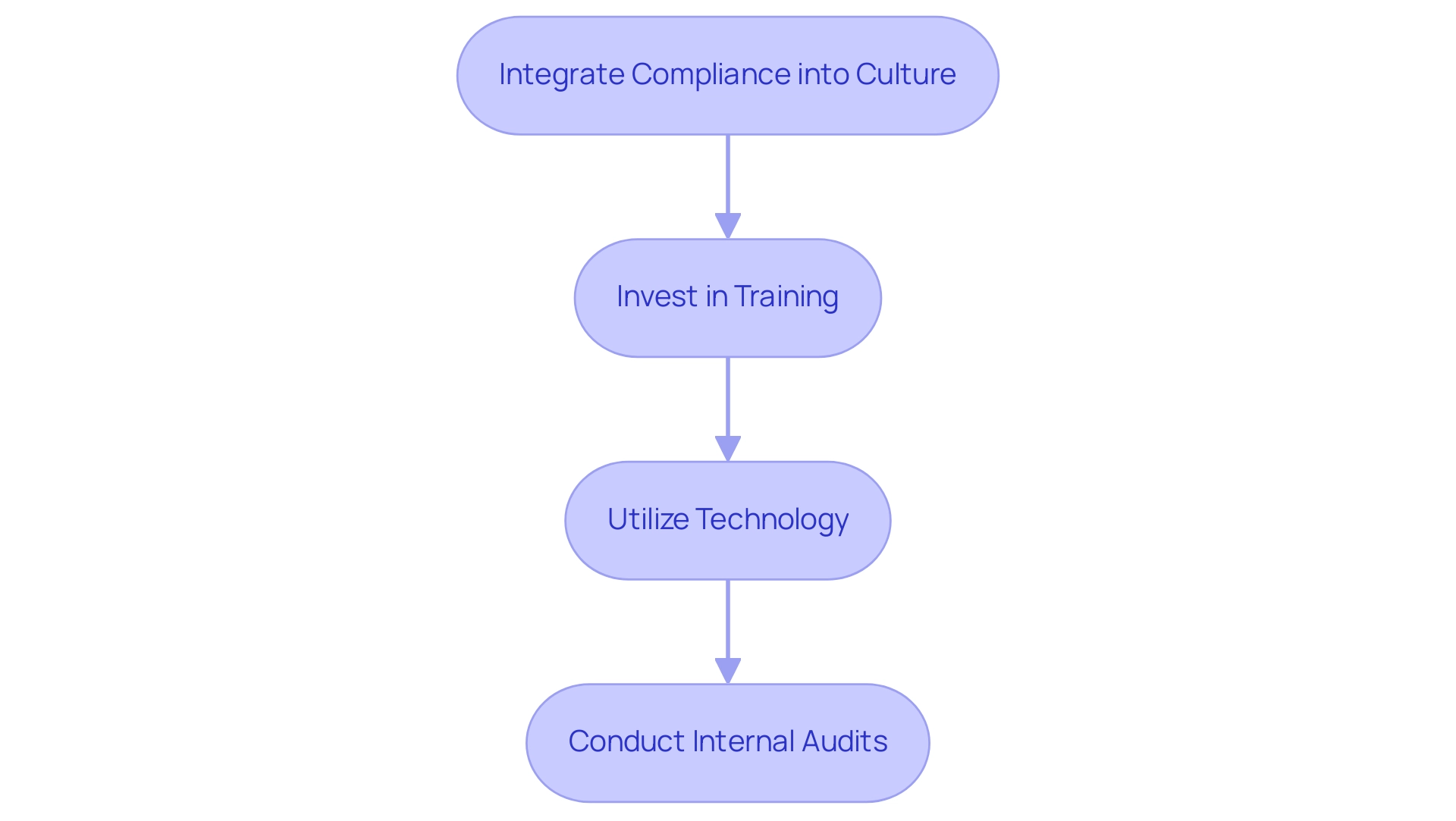
- Integrate Compliance into Organizational Culture: Cultivating a culture where compliance is viewed as a collective responsibility among all team members enhances accountability and fosters a proactive approach to regulatory adherence. As highlighted in the IMDRF guidelines on AI in medical devices, standardization in regulation is becoming increasingly crucial.
- Invest in Training: Regular and comprehensive training sessions focused on GSPR medical devices standards and the latest regulatory updates are vital. This commitment to education empowers your team with the knowledge necessary to navigate regulatory challenges effectively. According to industry insights, investing in training related to GSPR medical devices is essential for fostering a compliant organizational culture.
- Utilize Technology: Implementing advanced software solutions for document management and adherence tracking not only streamlines processes but also ensures real-time audit readiness. Tools such as Greenlight Guru Quality can be essential in reaching this objective, as they enable continuous monitoring of regulations.
- Conduct Internal Audits: Periodic internal audits are essential for evaluating the effectiveness of regulatory processes. These evaluations assist in recognizing possible areas for enhancement, ensuring that your organization stays aligned with legal expectations and can quickly adjust to any shifts in the oversight environment.
Nick Tippmann from MedTech Intelligence emphasizes, "4 Red Flags Investors Look For When Vetting Your Medical Device Company," highlighting the significance of strong regulatory frameworks in attracting investment.
By adhering to these optimal methods, healthcare product firms can enhance their adherence frameworks and improve readiness for the changing oversight landscape in 2024 and beyond. The importance of these practices is highlighted by the article's position as number 14650 in volume 14, issue 21 of the journal, indicating the essential nature of adherence in the healthcare equipment sector.
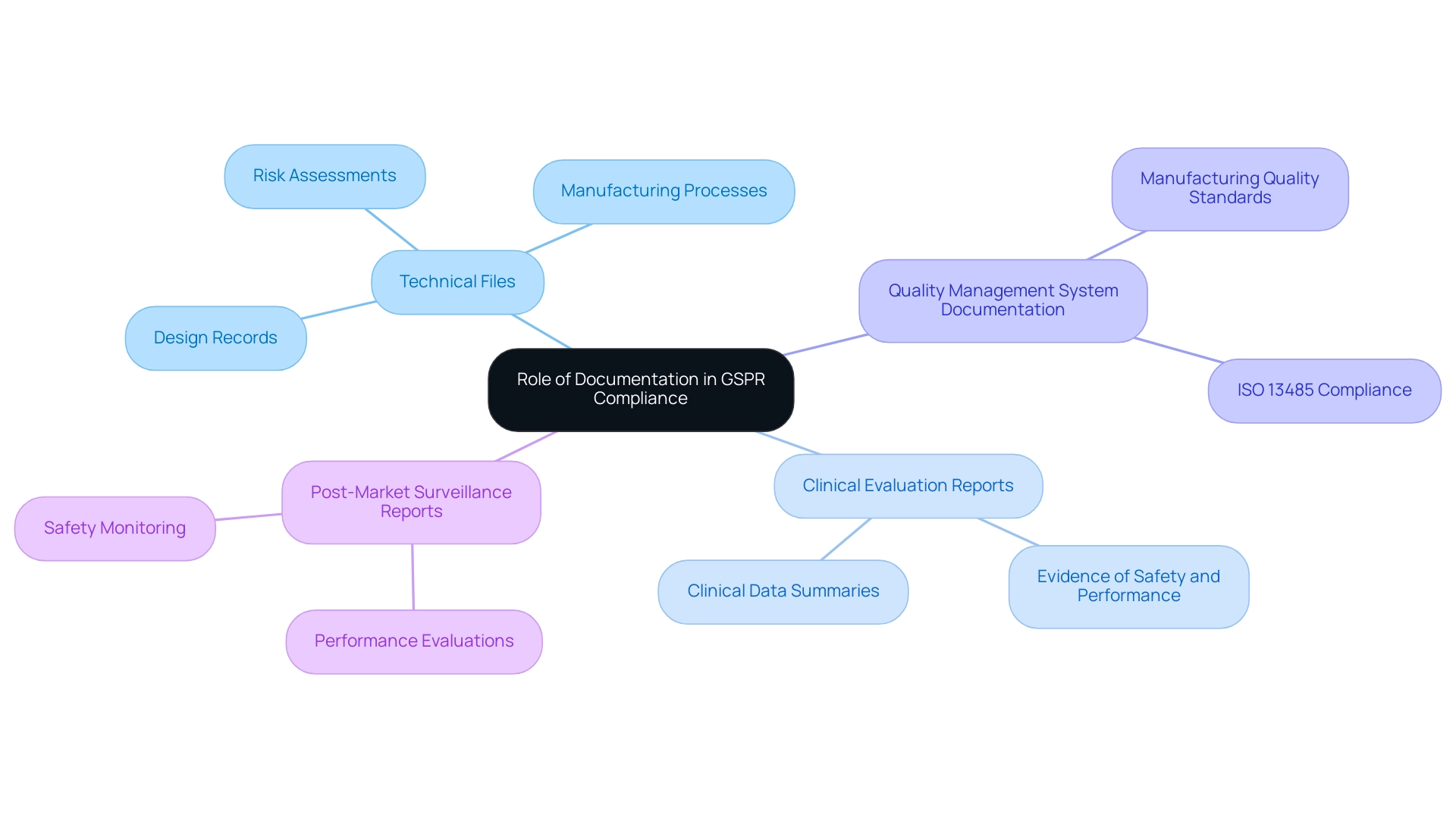
The Role of Documentation in GSPR Compliance
Documentation is integral to achieving conformity with the GSPR medical devices, as it serves as a cornerstone for regulatory adherence. As Peter Sebelius, a trainer and consultant in the medical device sector, emphasizes, "Effective documentation practices are crucial for ensuring that devices meet regulatory standards and maintain safety and performance throughout their lifecycle." In the context of clinical trials, our comprehensive management services encompass feasibility studies, site selection, regulatory reviews, trial setup, import permits, ongoing project management, and detailed reporting on study status and adverse events.
These services are essential in ensuring that documentation aligns with regulatory requirements and supports adherence throughout the trial process. The essential types of documentation include:
- Technical Files: Comprehensive records detailing the device's design, manufacturing processes, and risk assessments, which are critical for demonstrating compliance.
- Clinical Evaluation Reports: Summaries of clinical data that provide evidence of the product's safety and performance, affirming its efficacy in real-world applications.
- Quality Management System Documentation: Documents that confirm adherence to ISO 13485 standards, ensuring that the manufacturing processes meet industry benchmarks for quality.
- Post-Market Surveillance Reports: Continuous evaluations of the device's performance and safety after market introduction, which are vital for identifying any potential issues that may arise.
Keeping organized and up-to-date documentation is crucial for facilitating audits and effectively demonstrating compliance to authorities. Considering the implications of past incidents like the French PIP breast implant scandal, which took place around 10 years ago and prompted significant policy changes, the importance of strong documentation practices cannot be overstated. Many regulatory professionals are now enhancing their technical documentation for Class I and IIa products, yet a considerable number have not adequately tested their documentation under the new Medical Device Regulation (MDR).
Therefore, ensuring that technical files for GSPR medical devices are comprehensive and reflect current standards is essential for navigating the complexities of medical device regulation in 2024. For instance, the 4EasyReg GSPR Checklist serves as a customizable tool that aids in demonstrating compliance with the safety and performance requirements outlined in the EU regulations, exemplifying how effective documentation can streamline the compliance process.
Conclusion
Achieving compliance with the General Safety and Performance Requirements (GSPR) is crucial for medical device manufacturers aiming to ensure patient safety while fostering innovation in the healthcare sector. This article has explored the systematic approach necessary for GSPR compliance, highlighting essential steps such as:
- Conducting thorough risk assessments
- Developing clinical evaluation plans
- Maintaining robust technical documentation
Each of these components plays a vital role in not only meeting regulatory standards but also in enhancing the overall safety and efficacy of medical devices.
Moreover, the challenges faced by manufacturers in navigating the complexities of GSPR compliance cannot be understated. From keeping abreast of regulatory changes to managing resource constraints and engaging effectively with Notified Bodies, manufacturers must adopt a proactive and informed approach. By implementing best practices, such as:
- Fostering a culture of compliance
- Investing in ongoing training
organizations can better prepare themselves to meet the evolving regulatory landscape.
Documentation emerges as a cornerstone of GSPR compliance, underscoring its importance in facilitating regulatory adherence and ensuring device safety throughout their lifecycle. As the industry moves towards stricter regulations and heightened scrutiny, the emphasis on meticulous documentation practices will only grow stronger. By prioritizing compliance and leveraging expert guidance, manufacturers can navigate the complexities of the regulatory environment, ultimately contributing to safer medical devices and improved patient outcomes. The commitment to adhering to GSPR not only benefits individual organizations but also enhances the overall integrity of the medical device industry.
Frequently Asked Questions
What are GSPR medical devices?
GSPR medical devices refer to the General Safety and Performance Requirements mandated by the Medical Product Regulation (MDR) that healthcare products must meet to ensure their safety and efficacy.
Why are GSPR requirements important?
GSPR requirements are crucial for safeguarding public health and fostering innovation within the healthcare technology sector.
What key benchmarks are included in GSPR?
The key benchmarks in GSPR include risk management, clinical evaluation, and comprehensive post-market surveillance.
What is required from manufacturers regarding post-market studies?
Manufacturers are required to make the results of post-market equipment studies publicly available in a timely manner to enhance transparency and accountability.
How do manufacturers navigate GSPR compliance?
Manufacturers must understand GSPR to navigate compliance intricacies and ensure market access, often collaborating with one of approximately 80 for-profit, private Notified Bodies for assessment and CE marking.
What role does the FDA play in post-market surveillance?
The FDA conducts stringent post-market surveillance activities, including adverse event reporting and ongoing assessments, to monitor the safety of medical devices once they are on the market.
What are the steps to achieve GSPR compliance?
The steps to achieve GSPR compliance include conducting a risk assessment, developing a clinical evaluation plan, creating technical documentation, implementing quality management systems, engaging Notified Bodies, preparing for post-market surveillance, and leveraging clinical trial management services.
What is the significance of ISO standards in GSPR compliance?
Compliance with ISO 14971 and ISO 13485 standards is vital for establishing a solid risk management system and ensuring product quality in medical product manufacturing.
How can clinical trial management services assist in GSPR compliance?
Clinical trial management services can streamline the GSPR adherence process by providing support in areas such as feasibility studies, site selection, trial setup, and regulatory reviews.
What is INVIMA's role in the context of GSPR compliance in Colombia?
INVIMA plays a vital role in health product supervision and categorization as a Level 4 health authority by PAHO/WHO, helping manufacturers navigate local compliance frameworks.




