Overview
The article focuses on how to effectively align clinical research goals with business objectives, emphasizing strategies for organizations to integrate their research initiatives with broader commercial aims. It supports this by outlining a step-by-step guide that includes identifying key organizational objectives, mapping initiatives to these goals, engaging stakeholders, and measuring success through defined performance indicators, thereby illustrating the importance of strategic alignment in enhancing both research outcomes and business performance.
Introduction
In the realm of clinical research, the intersection with business objectives has never been more critical. As the number of registered trials continues to rise—over 453,000 in 2023—organizations must navigate the complexities of aligning their research initiatives with strategic business goals. This alignment is particularly vital for Medtech companies operating in dynamic markets like Latin America, where regulatory challenges and stakeholder engagement play pivotal roles in success.
By integrating robust clinical trial management services and fostering collaboration among diverse stakeholders, organizations can not only enhance patient outcomes but also drive significant business performance. This article delves into the strategies and frameworks necessary for achieving this alignment, providing insights into effective practices that can bolster both clinical research and business objectives in today's competitive landscape.
Understanding the Intersection of Clinical Research and Business Goals
Understanding how to align clinical research goals with business objectives starts with a thorough comprehension of the intersection between healthcare studies and corporate goals. In 2023, the landscape of medical studies revealed a total of 453,803 registered trials, underscoring the expansive role of medical inquiry in driving innovation and informing product development. Successful medical studies not only improve patient results but also greatly impact business performance, especially for Medtech firms operating in the Latin American market.
For example, the CRASH trial marketing approach concentrated on involving healthcare professionals instead of patients, emphasizing the significance of stakeholder dedication in reaching study objectives. Furthermore, the unique challenges faced by Medtech firms, such as regulatory hurdles and language barriers, necessitate robust collaboration and comprehensive clinical trial management services. These services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Organizations must critically assess how their investigative initiatives can strengthen wider commercial goals, including market expansion, revenue growth, and establishing a competitive advantage, which is essential in understanding how to align clinical research goals with business objectives. This strategic alignment necessitates a comprehensive evaluation of ongoing initiatives to ensure they align with the principles of How to Align Clinical Research Goals with Business Objectives, ultimately driving overall success. The aim of strong studies is to establish a solid evidence foundation for patient care, which is vital for understanding how to align clinical research goals with business objectives.
As noted by MKC, a Principal Investigator, 'The key implication for clinicians is that insufficient attention to management issues and marketing or sales activities will degrade the performance of the trial.' This highlights the importance of combining medical studies initiatives with strong commercial strategies to understand how to align clinical research goals with business objectives to enhance impact. Furthermore, recognizing that numerous clinical variables might exhibit nonlinear connections, as highlighted by recent conversations on linear regression, introduces complexity to the relationship between clinical studies and organizational performance, which emphasizes the necessity of understanding how to align clinical research goals with business objectives.
Step-by-Step Strategies for Aligning Research with Business Objectives
- Identify Key Organizational Objectives: Begin by thoroughly defining the core objectives that your organization aims to achieve. These may encompass goals such as increasing market share, enhancing patient satisfaction, or refining product offerings to meet evolving market demands.
- Map Initiatives to Objectives: Conduct a detailed analysis of both current and prospective projects to identify how they can effectively support the established organizational goals. Utilize extensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, and review and feedback on study documents, to learn how to align clinical research goals with business objectives. Create a mapping document that clearly associates each initiative with specific organizational objectives, ensuring clarity in purpose and direction.
- Develop Collaborative Plans: Create cooperative plans that actively engage both academic and commercial teams. This may involve arranging joint meetings to promote open discussions about progress, challenges, and necessary adjustments to maintain alignment with organizational goals. As emphasized in recent industry trends, 33% of companies intend to begin utilizing Account-Based Marketing (ABM) in 2022, highlighting the necessity for strategic alignment between commercial and analysis efforts.
- Establish Metrics for Success: Formulate measurable success indicators that will assess the impact of initiatives on organizational objectives. Key performance indicators might include return on investment (ROI), patient outcomes, and market feedback, which are critical in assessing the effectiveness of alignment. Significantly, companies often deliver only 50-60% of the financial performance their strategies promise, as illustrated in the case study on low ROI on strategy execution. This emphasizes the importance of understanding how to align clinical research goals with business objectives in order to bridge that performance gap.
- Regularly Review and Adjust: Establish a systematic review process to continuously evaluate the alignment of research initiatives with organizational objectives. Regular assessments will enable prompt modifications to strategies based on feedback, emerging trends, and changing priorities, which is essential for understanding how to align clinical research goals with business objectives. As mentioned by Sarah A. Attaf EL Shemouty, an Operations Analyst at IFC, 'Communication is the key to ensure that the statistical goals are aligned with the organizational objectives,' emphasizing the importance of ongoing dialogue in achieving successful alignment. By integrating robust trial management services, including trial set-up, start-up, and reporting on study status, your organization can enhance its operational efficiency and contribute to job creation and healthcare improvements in local economies.
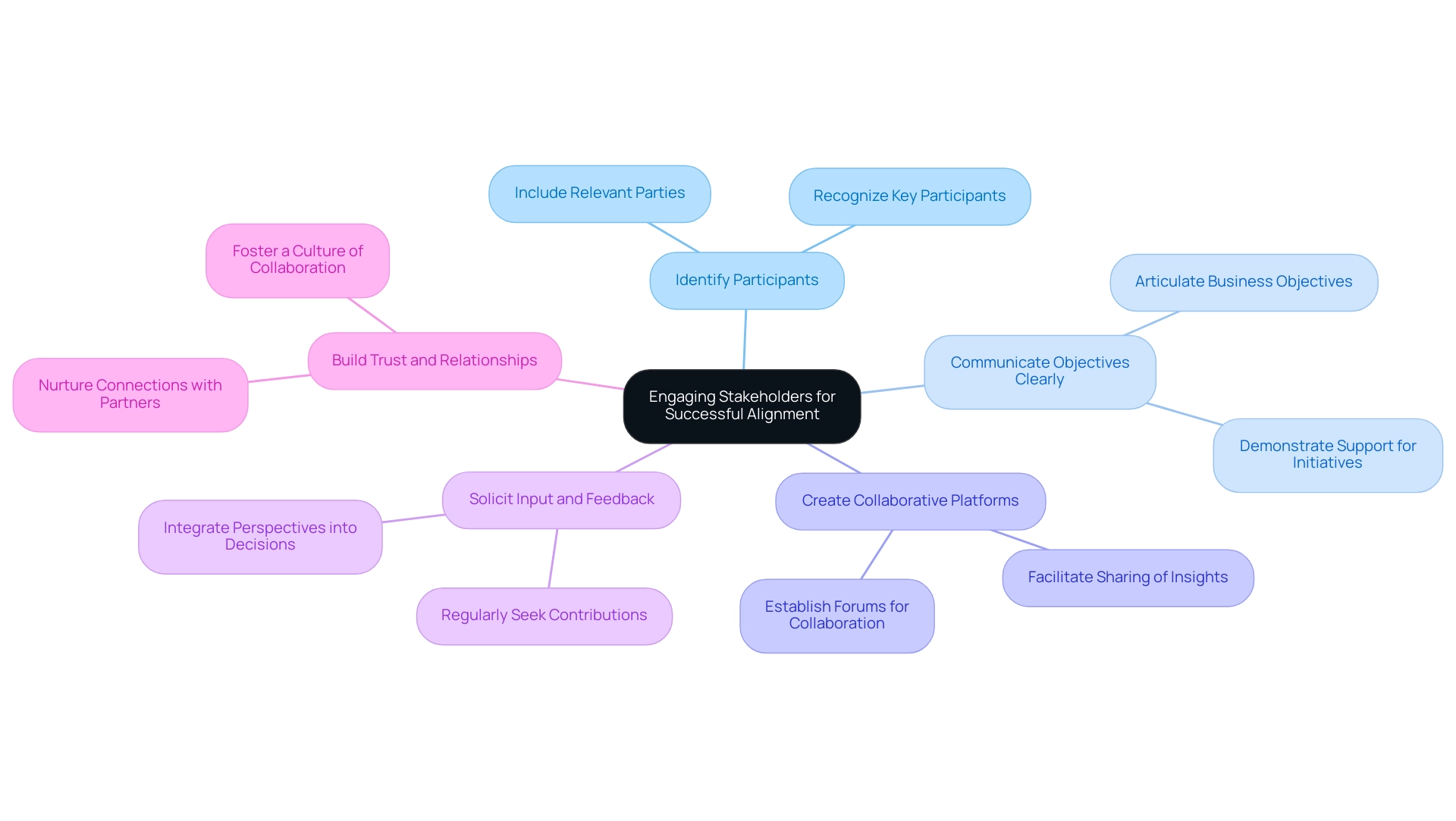
Engaging Stakeholders for Successful Alignment
Involving interested parties is essential for understanding how to align clinical research goals with business objectives. Important participants include researchers, industry leaders, regulatory bodies, and patient advocacy groups. As Laurel K Leslie, a medical doctor and public health expert linked to Tufts University and Tufts Medical Center, highlights, the participation of varied perspectives is essential in study alignment.
To foster effective engagement, consider the following strategies:
-
Identify Participants: Start by recognizing the key participants for each study initiative and organizational objective. This foundational step ensures that all relevant parties are included in the alignment process.
-
Communicate Objectives Clearly: Articulate business objectives transparently, demonstrating how investigative initiatives can support them. This clarity assists participants in comprehending their roles and contributions within the context of how to align clinical research goals with business objectives.
-
Create Collaborative Platforms: Establish forums or platforms that facilitate collaboration, allowing participants to share insights and provide feedback on research initiatives. These collaborative environments enhance participant involvement and investment.
-
Solicit Input and Feedback: Regularly seek contributions from interested parties to ensure their perspectives are integrated into decision-making processes. As a partner observed,
This study has enlightened me personally to how significant my contribution is
—emphasizing the essential role of participant voices in influencing study results.
-
Build Trust and Relationships: Invest time in nurturing connections with partners, fostering a culture of collaboration and mutual respect. This relational investment is vital for ongoing involvement and alignment.
Our extensive trial management services, including feasibility studies, site selection, compliance reviews, and reporting processes, contribute to the successful execution of projects while creating jobs and stimulating economic growth in local communities. Current trends highlight how to align clinical research goals with business objectives, emphasizing the growing acknowledgment of the significance of participant involvement in clinical studies. The MuSE Consortium, established through collaborative dialogues among study groups from Canada, the US, and the UK in 2015, exemplifies efforts to enhance participant engagement in studies and guidelines.
Furthermore, case studies have indicated future inquiry directions concentrating on comprehending the essence of participant involvement, assessing its influence on study results, and creating tools for more efficient engagement practices. Significantly, the Altmetric score of a recent article, which ranked it in the top 5% of all medical articles, highlights the increasing focus on effective stakeholder collaboration and its positive impact on study outcomes, illustrating the importance of our compliance reviews and project management in promoting this engagement.
Navigating Regulatory Frameworks to Support Alignment
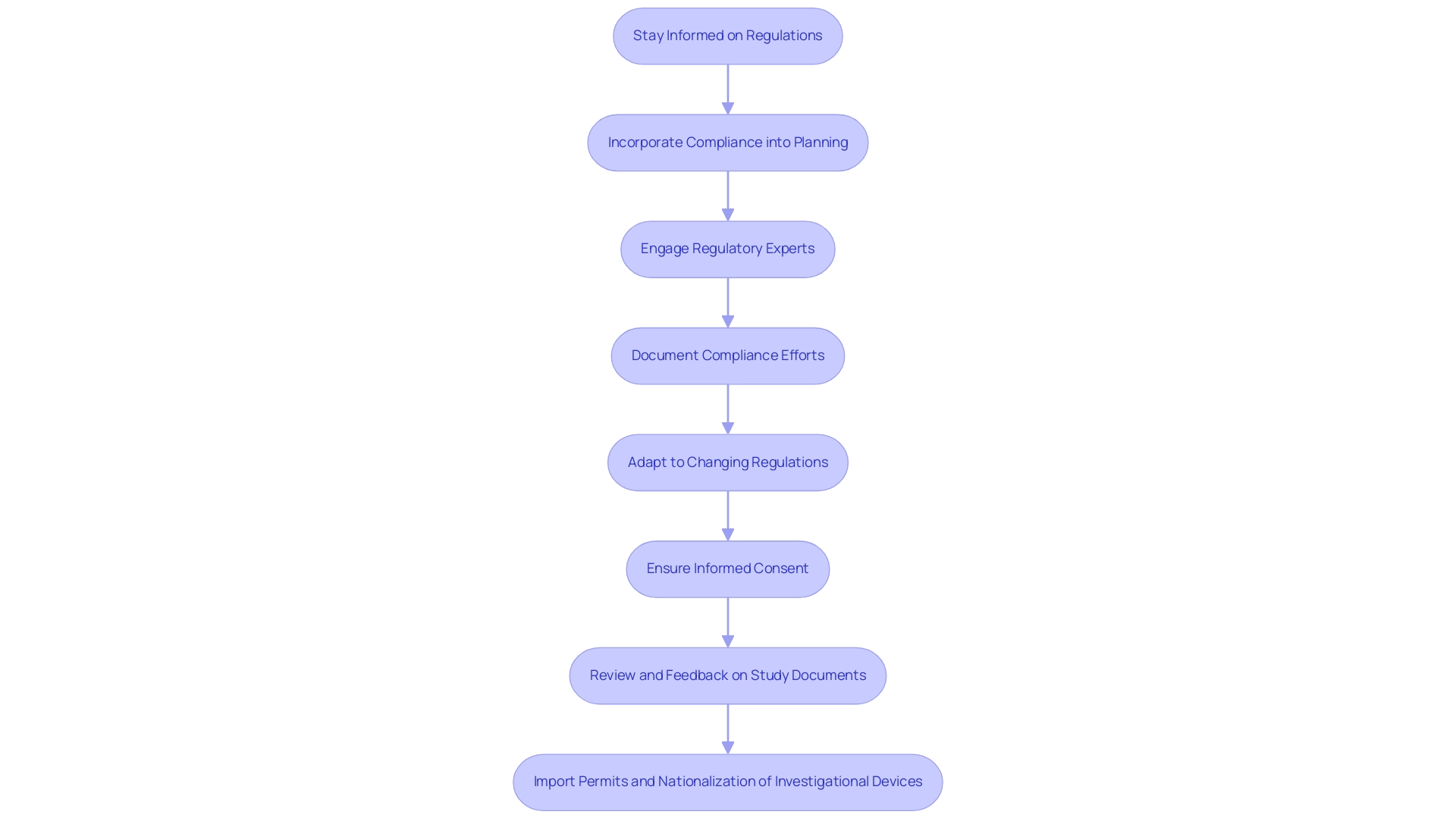
Navigating regulatory frameworks is crucial for understanding how to align clinical research goals with business objectives. Organizations must ensure that their investigative initiatives adhere to all pertinent regulations established by bodies such as the FDA and EMA. Here are several critical steps for effectively managing this regulatory landscape:
-
Stay Informed on Regulations:
Continuously update your understanding of the evolving rules and guidelines affecting medical studies. This knowledge is vital as an Investigational New Drug (IND) application automatically becomes effective 30 calendar days post-receipt, unless the FDA imposes a clinical hold.
-
Incorporate Compliance into Planning:
Embed compliance considerations from the outset of projects. This proactive approach assists in addressing all regulatory aspects early on, including the requirement for institutions involved in non-exempt human subjects studies to submit a written assurance of compliance to the Office for Human Research Protections (OHRP).
-
Engage Regulatory Experts:
Involve regulatory specialists during both the planning and execution phases of the study. Their insights are crucial for ensuring adherence to all requirements, particularly in complex situations where guidance may vary, as noted by industry experts:
This is very much an 'it depends' situation where there is no specific guidance.
-
Document Compliance Efforts:
Maintain comprehensive documentation of compliance activities, which will be invaluable during audits and reviews. Accurate records reinforce your organization's commitment to regulatory adherence.
-
Adapt to Changing Regulations:
Stay agile by being prepared to adjust study strategies in response to regulatory updates. This adaptability is critical for maintaining compliance and ensuring how to align clinical research goals with business objectives.
-
Ensure Informed Consent:
For studies involving human specimens that can be linked to specific individuals, be mindful of the informed consent requirements. Broad consent may also be applicable for secondary studies, enhancing the ethical framework of your initiatives.
-
Review and Feedback on Study Documents:
Ensure that all study documents are thoroughly reviewed and feedback is provided to comply with country requirements, which is crucial for maintaining regulatory compliance.
-
Import Permits and Nationalization of Investigational Devices:
Be aware of the import permit processes and nationalization requirements for investigational devices, as these are essential for legal compliance in research trials. Consider the case of the Spotlight Process, where Julia Black emphasizes the advantages of regulatory frameworks centered on risk. Such approaches promote greater coherence in regulatory practices, ultimately benefiting both compliance and effectiveness.
By implementing these strategies alongside extensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and thorough reporting—organizations can navigate the complexities of regulatory frameworks while achieving their scientific objectives.
Measuring Success: Evaluating Alignment Outcomes
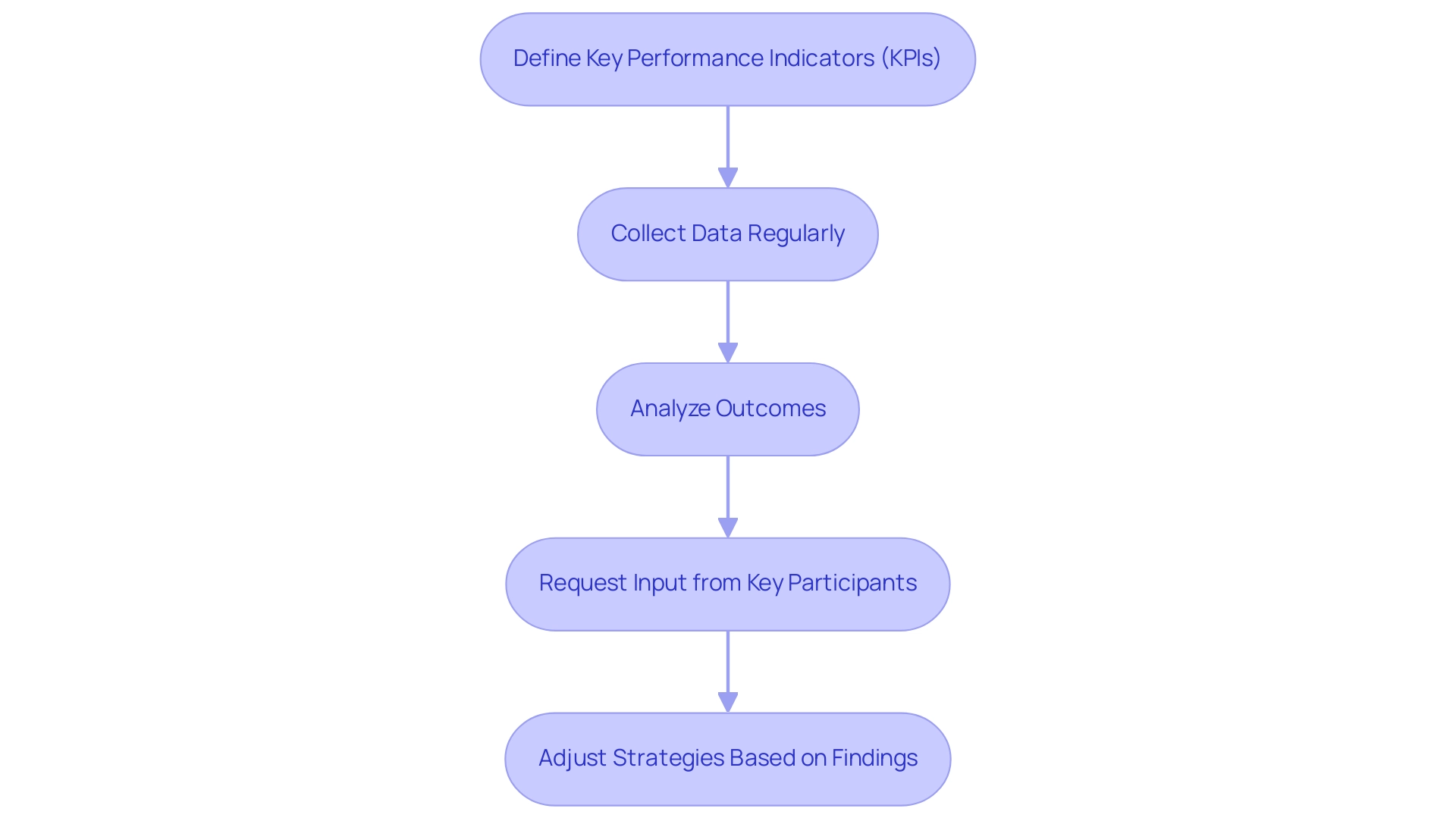
Measuring success involves understanding how to align clinical research goals with business objectives through a meticulous approach that evaluates both qualitative and quantitative outcomes. Here are essential steps to effectively gauge this alignment:
- Define Key Performance Indicators (KPIs): Establishing KPIs is crucial as they serve as benchmarks for evaluating the success of initiatives. Effective KPIs may include metrics such as patient recruitment rates, which are critical given that 134 irrelevant publications were removed from a recent review, leaving only 70 pertinent studies. This emphasizes the need for targeted recruitment strategies. Other potential KPIs include publication success rates and financial returns from investigative activities. Furthermore, the evaluation process can be customized extensively to fit the specific needs of different research initiatives, allowing for a more tailored approach to measuring success.
- Collect Data Regularly: Implementing a systematic approach for regular data collection is vital. This process not only tracks the defined KPIs but also enables timely interventions based on emerging trends and insights, which are essential for successful project management and oversight in trials. bioaccess™ excels in this area by offering comprehensive project management services that facilitate ongoing data collection and analysis.
- Analyze Outcomes: Conducting thorough analysis of the collected data is essential to assess the effectiveness of alignment efforts. For instance, Clariness has set ambitious standards for patient engagement, aiming for initial contact within 20 minutes in the US and scheduling visits within 10 days. These benchmarks illustrate the significant correlation between timely communication and patient randomization success, reinforcing the need for comprehensive data analysis. In partnership with Caribbean Health Group, bioaccess™ has shown a capacity to improve recruitment rates, attaining over a 50% decrease in recruitment time and a 95% retention rate, highlighting the effect of effective study management.
- Request Input from Key Participants: Collecting feedback from important contributors, including medical teams and institutional partners, offers valuable insights into the perceived success of alignment initiatives. This feedback can reveal areas requiring improvement and inform future strategies, especially in the context of international collaboration fostered by initiatives supported by Colombia's Minister of Health. The feedback process is integral to bioaccess™'s service offerings, as it helps refine trial setup and compliance reviews to better meet stakeholder needs.
- Adjust Strategies Based on Findings: Utilizing evaluation outcomes to refine and modify strategies ensures that medical studies continue to effectively support overarching organizational objectives. As articulated by Jithin Sreedharan from the University of Doha for Science and Technology, "By developing and implementing meaningful KPIs, institutions and policymakers can gain valuable insights into the performance of the sector." This iterative process not only enhances research alignment but also illustrates how to align clinical research goals with business objectives, driving continuous improvement in health outcomes and contributing to local economic growth and healthcare enhancement. bioaccess™'s commitment to compliance reviews and feasibility studies further supports this continuous improvement, ensuring that clinical trials are not only efficient but also aligned with business objectives.
Conclusion
The integration of clinical research with business objectives is paramount for organizations, particularly within the Medtech sector navigating the complexities of markets like Latin America. This article has outlined essential strategies for achieving this alignment, emphasizing the necessity of understanding the intersection between clinical research and business goals. Key steps include:
- Identifying core business objectives
- Mapping research initiatives accordingly
- Fostering collaboration among stakeholders
Successful alignment hinges on effective stakeholder engagement and navigating regulatory frameworks. By actively involving diverse voices and maintaining compliance with evolving regulations, organizations can enhance their research initiatives while simultaneously addressing business performance. Regular evaluation of progress and adapting strategies based on measurable outcomes further solidifies this alignment, ensuring that clinical research contributes to broader organizational success.
In conclusion, the pursuit of aligning clinical research with business objectives is not merely a strategic advantage but a fundamental requirement in today's competitive landscape. By embracing a structured approach to integration, organizations can not only improve patient outcomes but also achieve significant business growth, thereby reinforcing their position in the market. The commitment to this alignment will ultimately drive innovation and effectiveness in both clinical and commercial endeavors.
Frequently Asked Questions
What is the importance of aligning clinical research goals with business objectives?
Aligning clinical research goals with business objectives is crucial as it ensures that medical studies improve patient outcomes while also enhancing business performance, particularly for Medtech firms in competitive markets like Latin America.
How many registered clinical trials were there in 2023?
In 2023, there were a total of 453,803 registered clinical trials, highlighting the significant role of medical inquiry in innovation and product development.
What challenges do Medtech firms face in aligning clinical research with business goals?
Medtech firms encounter unique challenges such as regulatory hurdles and language barriers, which require robust collaboration and comprehensive clinical trial management services to address effectively.
What are some key clinical trial management services mentioned?
Key clinical trial management services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What steps can organizations take to align their research initiatives with commercial goals?
Organizations can take several steps, including identifying key organizational objectives, mapping initiatives to objectives, developing collaborative plans, establishing metrics for success, and regularly reviewing and adjusting strategies.
Why is stakeholder dedication important in clinical research?
Stakeholder dedication is vital as it helps to effectively reach study objectives, as demonstrated by the CRASH trial marketing approach, which focused on engaging healthcare professionals.
How can organizations measure the success of their initiatives?
Organizations can measure success through key performance indicators such as return on investment (ROI), patient outcomes, and market feedback.
What role does communication play in aligning clinical research with business objectives?
Communication is essential for ensuring that statistical goals are aligned with organizational objectives, allowing for ongoing dialogue and adjustments based on feedback and emerging trends.
How can regular reviews impact the alignment process?
Regular reviews enable organizations to continuously evaluate the alignment of research initiatives with objectives, allowing for prompt modifications to strategies based on changing priorities and emerging trends.




