Overview
Building successful trial partnerships in Colombia necessitates a strategic understanding of the local Medtech landscape. This includes:
- Engaging with key stakeholders
- Navigating regulatory compliance
- Tailoring recruitment strategies to cultural contexts
The importance of collaboration with local entities is paramount, as is the utilization of technology to enhance trial efficiency. Successful examples, such as the partnership between Bioaccess and Caribbean Health Group, showcase how these strategies significantly improved recruitment outcomes in clinical studies.
Introduction
In the rapidly evolving landscape of medical technology, Colombia is emerging as a vital hub for clinical research and innovation. This dynamic environment, characterized by a diverse patient population and a regulatory framework increasingly supportive of Medtech initiatives, presents a wealth of opportunities for organizations seeking to establish successful trial partnerships.
Key factors driving this growth include:
- Robust market expansion
- The significance of local collaborations
- A profound understanding of cultural contexts that can enhance recruitment strategies
As stakeholders navigate the complexities of regulatory compliance and strive to leverage technology for improved trial efficiency, the insights derived from this landscape can pave the way for successful clinical trials and effective market access.
Understanding the Colombian Medtech Landscape
Building trial partnerships in Colombia necessitates a thorough understanding of the Medtech landscape to achieve success. This nation is emerging as a prominent hub for medical technology, characterized by a diverse patient population and a regulatory environment that increasingly supports clinical research initiatives. Key considerations include:
-
Market Growth: The Colombian Medtech market is on a robust growth trajectory, with projections indicating significant expansion driven by rising healthcare demands and rapid technological advancements. By 2025, the market is expected to witness a substantial increase, reflecting the country's commitment to enhancing healthcare delivery. Insights from the Horizon Databook reveal that the medical device outsourcing market in Colombia is poised for growth, presenting opportunities for collaboration and investment.
-
Key Players: It is essential to identify and engage with local manufacturers, distributors, and research institutions that are integral to the Medtech ecosystem. Collaborating with these stakeholders can provide valuable insights and facilitate smoother testing processes. Notably, ReGelTec's recent Early Feasibility Study in Barranquilla, where eleven patients were successfully treated with HYDRAFIL™, exemplifies the potential for innovative treatments in this region.
-
Cultural Context: A profound comprehension of local healthcare practices and patient preferences is essential for enhancing recruitment strategies and study design. Tailoring approaches to align with cultural nuances can significantly enhance participant engagement and retention. The partnership between Bioaccess and Caribbean Health Group is focused on building trial partnerships in Colombia to establish Barranquilla as a premier location for medical studies in Latin America, supported by the Health Minister of the nation, which emphasizes the importance of cultural harmony in the success of research.
-
Economic Factors: Evaluating the economic landscape, including potential funding opportunities and partnerships with local stakeholders, is critical for ensuring efficient trial execution. The increasing need for innovative medical devices, combined with the country's well-developed healthcare infrastructure in urban regions, creates a favorable setting for medical research. The case analysis titled 'Healthcare Infrastructure and Disease Burden' demonstrates that the significant burden of chronic and infectious illnesses in the country is raising the demand for new medical devices, thus enhancing the need for outsourcing service providers. Furthermore, building trial partnerships in Colombia has enabled GlobalCare Clinical Trials' collaboration with Bioaccess to achieve over a 50% reduction in recruitment time and a 95% retention rate, showcasing the effectiveness of strategic collaborations.
-
Bioaccess Services: Bioaccess provides extensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. These services are crucial for managing the intricacies of research studies in Colombia.
-
INVIMA Overview: The Colombian National Food and Drug Surveillance Institute (INVIMA) plays a crucial role in regulating medical devices and ensuring compliance with health standards. As a Level 4 health authority acknowledged by PAHO/WHO, INVIMA's supervision is essential for preserving the integrity of medical research in the country.
In conclusion, utilizing these insights can enable organizations to efficiently traverse the Colombian Medtech environment, ultimately resulting in successful research phases and market entry. As noted by MarkWide Research, "Their reports are thorough, accurate, and delivered on time. We appreciate their professionalism and expertise, and would highly recommend their services to other companies looking for reliable market research," emphasizing the importance of having reliable market insights for informed decision-making.
Navigating Regulatory Compliance in Colombia
To successfully navigate regulatory compliance in Colombia, it is imperative to follow these essential steps:
- Understand INVIMA Requirements: The National Food and Drug Surveillance Institute (INVIMA) serves as the regulatory body overseeing clinical trials in Colombia. Familiarizing yourself with their comprehensive guidelines and submission processes is crucial to ensure compliance and facilitate a smoother approval process.
- Prepare Necessary Documentation: Meticulously prepare all required documents, including research protocols, informed consent forms, and ethical approvals. Proper documentation is essential, as it not only aids the approval process but also enhances the credibility of your research.
- Engage with Local Ethics Committees: Collaborating with local ethics committees is vital to obtain the necessary approvals. This involvement prioritizes participant rights and safety, ensuring that ethical standards are maintained throughout the process. Key ethical factors in INVIMA's approval process include informed consent, participant safety, ethics committee review, special considerations for vulnerable populations, and transparency in reporting research results.
- Monitor Regulatory Changes: The regulatory environment can shift swiftly, making it essential to stay informed about any modifications that may affect your study. Regularly reviewing INVIMA announcements and industry news will help you remain compliant and avoid potential setbacks.
- Utilize Local Expertise: Partnering with local contract research organizations (CROs) like bioaccess® can provide invaluable insights into navigating the Colombian regulatory environment. bioaccess® offers extensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. Their expertise can streamline the process and improve the chances of successful results.
As industry specialists emphasize, "Clear objectives are essential to the success of clinical studies." Colombia's regulatory framework is bolstered by its status as the sixth-largest contributor to research studies in Latin America, accounting for 5.9% of examinations in the region. Moreover, the country offers generous R&D tax incentives, including a 50% tax rebate on research investments, significantly benefiting Medtech companies aiming to innovate and expedite market access.
As of 2025, inter-trial intervals have improved to 17 months, a notable reduction from the pandemic peak of 32 months, reflecting a more efficient regulatory process. Understanding these dynamics will enable you to establish successful collaborative ventures, including building trial partnerships in Colombia. For additional assistance in navigating the regulatory environment and conducting medical studies in a South American country, consider engaging with bioaccess®.
Effective Recruitment Strategies for Clinical Trials
To implement effective recruitment strategies for clinical trials in Colombia, consider the following approaches:
-
Leverage Local Networks: Collaborate with local healthcare providers, hospitals, and clinics to access their patient networks. This engagement not only facilitates participant identification but also enhances trust, which is crucial for successful recruitment. As highlighted by industry leaders, building trial partnerships in Colombia is essential for navigating the complexities of the Latin American Medtech landscape.
-
Culturally Tailored Messaging: Create recruitment materials that resonate with the local population. This includes considering language preferences, cultural nuances, and varying levels of health literacy. Customized communication can greatly enhance participant involvement and readiness to enroll, ensuring that the study is viewed favorably within the community.
-
Utilize Digital Platforms: Harness the power of social media and online patient registries to broaden your reach. Digital platforms can effectively engage potential participants, especially younger demographics who are more active online, thus increasing enrollment rates. This approach aligns with the growing trend of digital involvement in medical research.
-
Encourage Involvement: Providing rewards like transportation reimbursement or free health check-ups can inspire individuals to engage in studies. This strategy has been shown to enhance recruitment success rates, particularly in underserved populations, and reflects a commitment to addressing barriers to participation.
-
Community Engagement: Establish connections with community leaders and organizations to cultivate trust and raise awareness about the research study. Involving the community not only improves recruitment efforts but also guarantees that the study is perceived favorably, which can result in increased participation rates. This community-focused strategy, building trial partnerships in Colombia, is essential for the success of medical studies in the country, as highlighted by specialists in the area.
In 2025, the emphasis on patient recruitment success rates in the country is paramount, as local healthcare networks play a critical role in participant recruitment. The support services market for studies in Argentina is expected to attain USD 249.8 million by 2030, suggesting considerable growth potential in the area that could also advantage Colombia. Furthermore, specialists stress the significance of enhancing outcome assessment in medical studies, especially via patient-reported results, as underscored in the case study named "Measuring Outcomes."
This focus on outcomes is essential for enhancing the evaluation of therapeutic efficacy and safety.
Moreover, as pharmaceutical firms pivot their attention toward fewer, high-value therapeutic sectors due to drug price discussions, as pointed out by Ariel Katz, the overall count of research studies may decline, making efficient recruitment strategies even more essential. Max Baumann's perspectives on the difficulties biotech encounters in a saturated market highlight the importance for research sponsors to manage these intricacies efficiently, ensuring adherence and promoting market entry for cutting-edge medical technologies.
At bioaccess, we specialize in offering extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting. Our proficiency in traversing the Latin American Medtech environment guarantees that your clinical evaluations are not only compliant but also set for success in the region.
Building Partnerships with Local Stakeholders
To establish effective partnerships with local stakeholders in the region, consider the following steps:
- Identify Key Stakeholders: Begin by mapping out potential partners, which may include hospitals, universities, research institutions, and patient advocacy groups. Understanding the landscape of these entities is crucial for successful collaboration.
- Engage Early: Initiate discussions with stakeholders at the beginning of the planning process. Early engagement helps align goals and expectations, fostering a collaborative environment that can lead to smoother project execution. For instance, bioaccess™ and Caribbean Health Group (CHG) have effectively collaborated to establish Barranquilla as a premier location for research studies in Latin America, with backing from Colombia's Minister of Health.
- Foster Collaborative Relationships: Build mutually beneficial relationships by providing value to your partners. This could involve sharing research findings, offering training opportunities, or collaborating on community outreach initiatives that enhance the visibility of the study. The collaboration between GlobalCare Clinical Trials and bioaccess™ illustrates how strategic partnerships can improve trial ambulatory services, achieving over a 50% decrease in recruitment time and 95% retention rates.
- Attend Local Conferences and Events: Actively participate in industry events to network with potential partners and gain insights into local trends and challenges. Such engagements not only broaden your professional network but also keep you informed about the evolving landscape of clinical research in the country. Notably, with an average of 6.0 CT scans performed per one million inhabitants in Colombia, there is a rising trend in diagnostic imaging that stakeholders should be aware of.
- Maintain Open Communication: Establish clear communication channels to facilitate ongoing collaboration. Frequent updates and feedback cycles are crucial to address any issues that may arise during the experiment, ensuring that all stakeholders remain aligned and engaged throughout the process. Leveraging the expertise of professionals such as Juan Cuya, MD, and Oswaldo Amaya, MD, who specialize in regulatory affairs and medical device trials, can further enhance communication and project management.
By adhering to these steps, organizations can efficiently navigate the intricacies of the Colombian healthcare sector, focusing on building trial partnerships in Colombia to utilize local expertise and resources to boost the success of research trials. Furthermore, as highlighted by Fortune Business Insights, factors such as technological advancements and rising product approvals are expected to drive the adoption of innovative solutions, making it imperative for partnerships to adapt to these trends. Additionally, considering the significance of investing in renewable energy and enhancing regulatory frameworks will align these partnerships with the country's broader objectives for carbon neutrality.
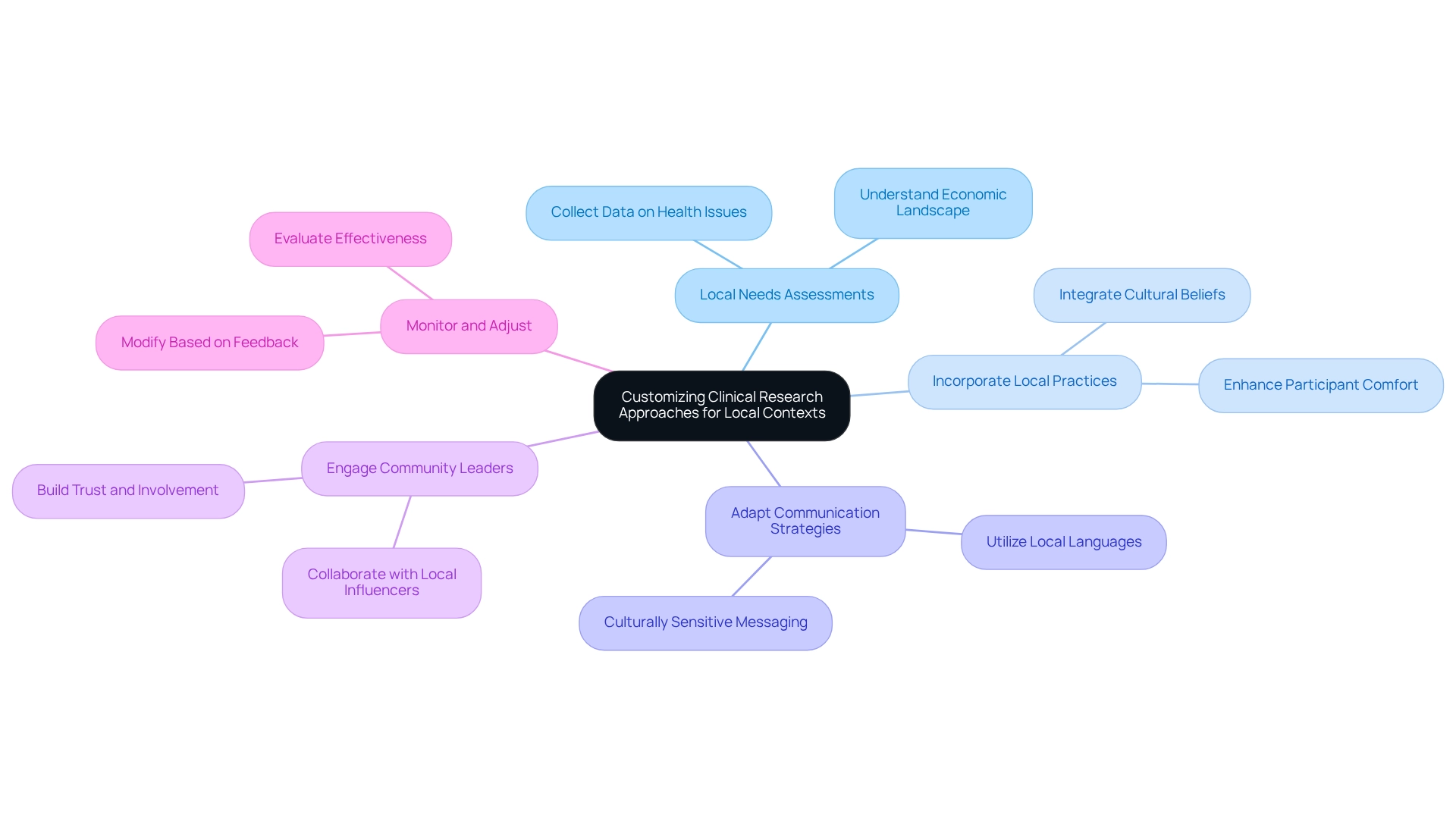
Customizing Clinical Research Approaches for Local Contexts
To effectively customize clinical research approaches for local contexts in Colombia, it is essential to implement the following strategies:
-
Conduct Local Needs Assessments: Collect comprehensive data on prevalent health issues, patient demographics, and healthcare access. With Colombia categorized as an upper-middle income (UMC) nation by the World Bank, comprehending the economic landscape is vital for creating studies that meet the particular needs of the population, ensuring relevance and effectiveness. The availability of high-quality national death registration data further supports the need for conducting thorough local needs assessments.
-
Incorporate Local Practices: Integrate culturally significant practices and beliefs into the research protocol. This not only enhances participant comfort but also fosters greater engagement and adherence to the study, ultimately improving outcomes.
-
Adapt Communication Strategies: Utilize local languages and culturally sensitive messaging in all trial-related communications. Clear and relatable communication is essential for ensuring participant comprehension and trust, which are crucial for successful study execution.
-
Engage Community Leaders: Collaborate with local leaders and influencers to gain insights into community dynamics. As illustrated by Esneda's activism and leadership, community engagement is essential. Her quote, "I became a leader because it was necessary. 'I was born to defend our land and people,'" highlights the importance of local leadership in building trust and involvement in clinical studies. Their participation can boost trust and enable smoother interactions with potential participants, making the study more acceptable and effective.
-
Monitor and Adjust: Continuously evaluate the effectiveness of your customized approaches. Be ready to make modifications based on participant feedback and the changing environment of the study. This adaptive strategy ensures that the research remains relevant and responsive to local needs.
By concentrating on these customized approaches, building trial partnerships in Colombia can lead to enhanced success and adherence in research studies, ultimately resulting in better market access for innovative medical technologies. Furthermore, the recent launch of the EU Country Cancer Profiles 2025 and the Country Health Profiles 2023 emphasizes the existing health landscape, stressing the significance of adjusting research to address local health challenges. Moreover, bioaccess's extensive service capabilities, including feasibility studies, setup, project management, and compliance reviews, play a crucial role in facilitating these clinical studies.
The successful case of ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain in that country exemplifies how tailored strategies can lead to effective outcomes in the region.
Leveraging Technology to Enhance Trial Efficiency
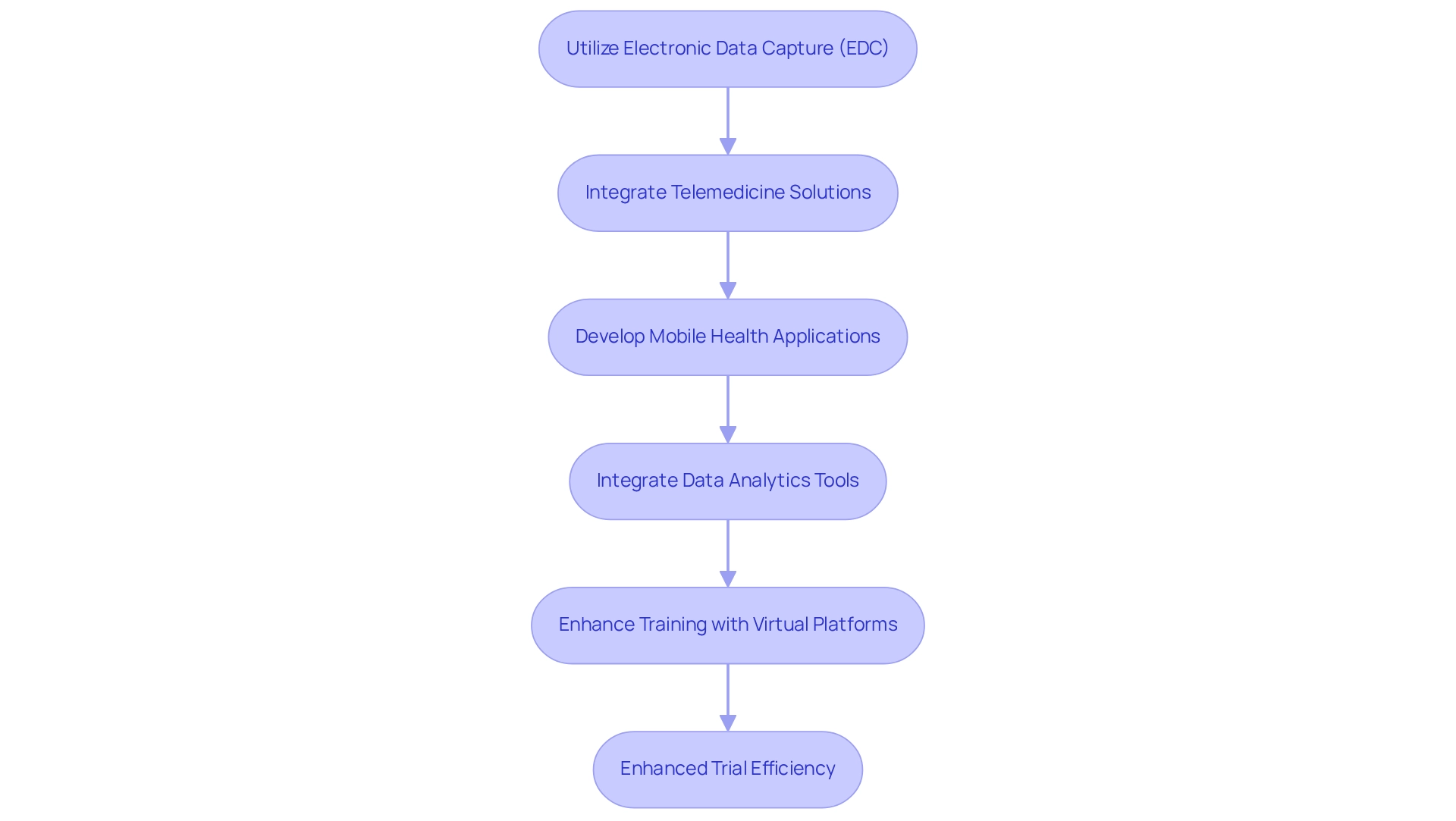
To enhance trial efficiency in Colombia through technology, consider implementing the following strategies:
-
Utilize Electronic Data Capture (EDC): EDC systems simplify data collection processes, significantly minimizing errors and allowing real-time monitoring of study data. This technology not only improves data integrity but also accelerates the overall testing timeline, which is essential in a competitive environment where the global top 10 pharmaceutical companies are valued above $3 trillion in combined market cap.
-
Integrate Telemedicine Solutions: By utilizing telemedicine for remote consultations and follow-ups, studies can enhance participant access and convenience. This approach is particularly beneficial in reaching diverse populations, ensuring that geographical barriers do not hinder participation.
-
Develop Mobile Health Applications: Creating mobile applications for participant engagement facilitates easy communication, sends reminders, and allows for seamless data submission. These apps can improve participant retention and compliance, ultimately resulting in more robust study outcomes.
-
Integrate Data Analytics Tools: Utilizing advanced analytics tools allows sponsors to monitor progress effectively, identify trends, and make informed, data-driven decisions. This proactive approach can lead to improved resource allocation and faster issue resolution. For example, some sponsors have utilized historical trend data to address challenges proactively, as demonstrated in the case analysis titled 'Proactive Issue Management Using Historical Data.' This approach has led to improved data quality and reduced project timelines, ultimately accelerating the time to market for clinical research.
-
Enhance Training with Virtual Platforms: Providing training for research staff and participants through virtual platforms ensures that all parties are well-prepared and informed. This method not only saves time but also allows for consistent training delivery, which is crucial for maintaining high-quality standards throughout the trial. As mentioned by the Head of Clinical Data Engineering, "Part of the initiative is to bring all our research in-house so that our internal teams can start working on it. They can be more practical, and we implement research internally and we are able to take charge of our data, and we provide for our patients with high quality."
By implementing these strategies, research organizations in Colombia, such as bioaccess, can significantly improve study efficiency, ultimately resulting in faster market access and better adherence to regulatory standards. This aligns with the growing focus on optimizing development journeys to achieve not only regulatory approval but also commercial success, while also contributing to local economies through job creation and healthcare improvements. Moreover, bioaccess's extensive service offerings, including viability assessments, site selection, and compliance evaluations, are essential in maneuvering through the regulatory environment overseen by INVIMA, guaranteeing that clinical assessments meet all required criteria and benefit the local economy.
The Role of Post-Market Studies in Medtech Success
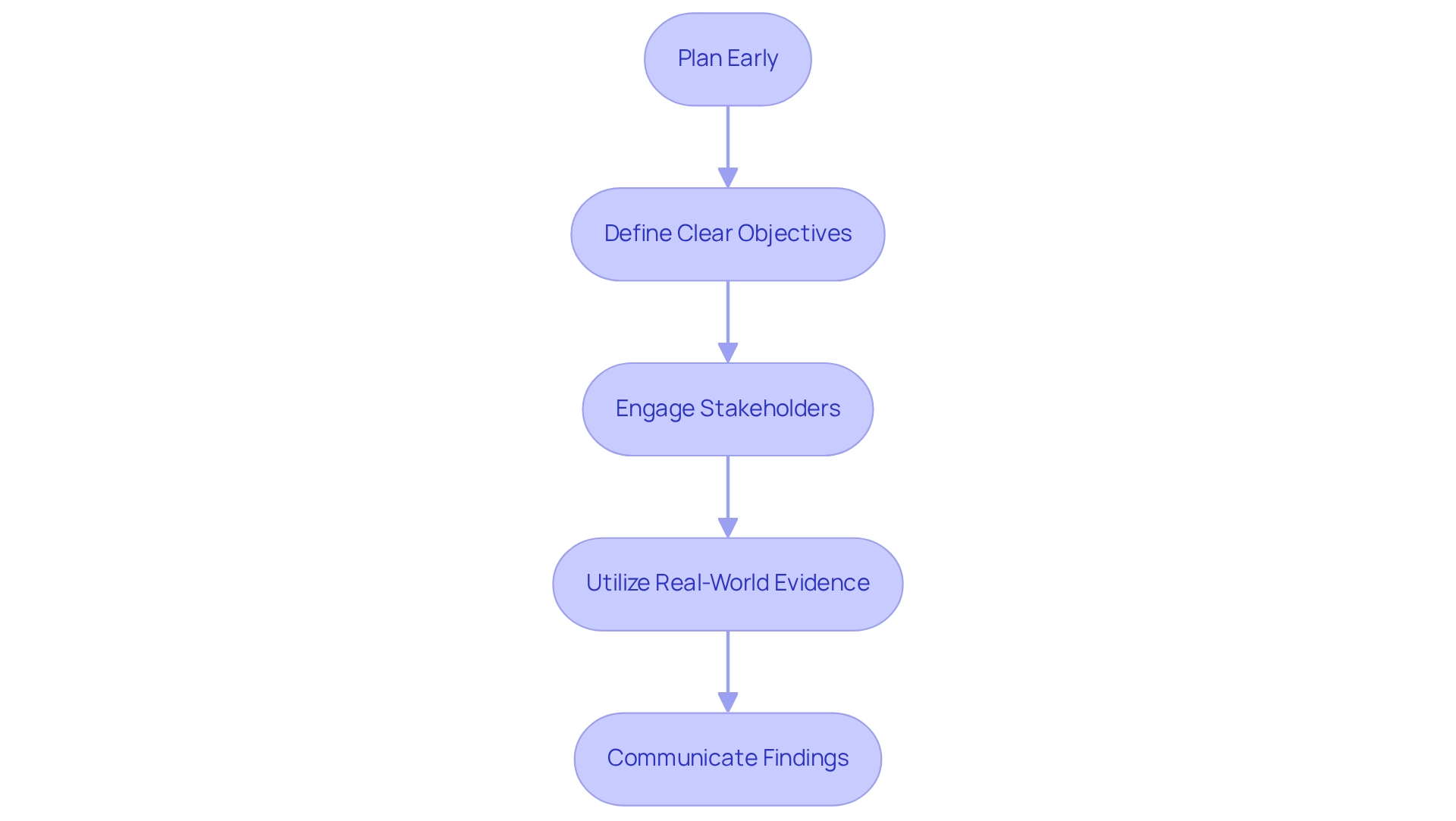
To effectively incorporate post-market studies into your Medtech strategy, consider the following key steps:
-
Plan Early: Incorporate post-market research considerations into the initial design. This proactive approach ensures alignment with regulatory requirements and facilitates smoother transitions from clinical trials to market entry.
-
Define Clear Objectives: Establish specific goals for post-market assessments, focusing on critical aspects such as long-term safety, effectiveness, and patient satisfaction. Clear objectives direct the research design and assist in evaluating success.
-
Engage Stakeholders: Collaborate with regulatory bodies, healthcare providers, and patients to gather comprehensive data and insights. Engaging these stakeholders early in the process fosters trust and enhances the quality of the data collected.
-
Utilize Real-World Evidence: Leverage real-world data to assess device performance across diverse populations and settings. This evidence is essential for understanding how devices operate outside regulated settings, offering insights that can guide future innovations.
-
Communicate Findings: Share results from post-market analyses with stakeholders to demonstrate a commitment to safety and ongoing enhancement. Transparent communication not only builds credibility but also reinforces the importance of ongoing monitoring in the Medtech sector.
In the nation, the importance of post-market research is highlighted by the country's strong clinical research environment, which ranks fourth in Latin America for clinical trials per million individuals, with a rate of 4.65. This environment is further enhanced by the recent success of ReGelTec's Early Feasibility Study, where eleven patients with chronic low back pain were treated with HYDRAFIL™. The procedures were proctored remotely via Zoom, and the hydrogel was injected into the nucleus of the degenerated disc via a 17-gauge needle, showcasing the potential for innovative treatments in the region.
Additionally, this country provides generous R&D tax incentives—allowing medtech companies to claim a 50% tax rebate on research investments—facilitating market entry for innovations, even those not yet FDA-approved. As noted by MarkWide Research, "Their reports are thorough, accurate, and delivered on time. We appreciate their professionalism and expertise, and would highly recommend their services to other companies looking for reliable market research."
As the orthopedic and cardiovascular device sectors are expected to expand considerably due to rising health issues, the incorporation of post-market research becomes even more vital in guaranteeing the safety and effectiveness of these devices in practical applications. Furthermore, bioaccess provides comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, reporting, and importantly, building trial partnerships in Colombia, which are essential for navigating the complexities of clinical trials.
Conclusion
The Colombian Medtech landscape offers a distinctive opportunity for organizations aiming to engage in clinical research and innovation. With robust market growth, a supportive regulatory environment, and a culturally diverse population, Colombia stands out as a key player in the global Medtech arena. Grasping the local context, which includes economic factors and healthcare practices, is crucial for establishing successful trial partnerships and optimizing recruitment strategies.
Navigating regulatory compliance through a comprehensive understanding of INVIMA requirements and engaging with local stakeholders can significantly streamline the clinical trial process. Implementing effective recruitment strategies that leverage local networks and culturally tailored messaging enhances participant engagement, ensuring higher enrollment rates and retention. Establishing strong partnerships with local entities not only fosters collaboration but also enriches the research process through shared insights and resources.
Technology plays a pivotal role in enhancing trial efficiency, from utilizing electronic data capture systems to integrating telemedicine solutions. By leveraging innovative tools, organizations can improve data integrity, participant access, and ultimately, the speed at which trials are conducted. Furthermore, incorporating post-market studies is vital for long-term success, providing valuable real-world evidence that informs device performance and patient satisfaction.
In conclusion, as Colombia continues to evolve as a Medtech hub, stakeholders must embrace the complexities and opportunities of the local landscape. By prioritizing collaboration, cultural alignment, and technological advancements, organizations can effectively navigate the intricacies of clinical trials, paving the way for successful market access and innovative healthcare solutions. The future of Medtech in Colombia is promising, and those willing to invest in understanding and adapting to this dynamic environment will reap the rewards of their efforts.
Frequently Asked Questions
What is the current state of the Medtech market in Colombia?
The Colombian Medtech market is experiencing robust growth, driven by rising healthcare demands and rapid technological advancements. By 2025, significant market expansion is expected, reflecting the country's commitment to improving healthcare delivery.
Why is it important to identify key players in the Colombian Medtech ecosystem?
Engaging with local manufacturers, distributors, and research institutions is essential for gaining valuable insights and facilitating smoother testing processes. Collaborations can enhance research outcomes, as demonstrated by ReGelTec's successful Early Feasibility Study in Barranquilla.
How does cultural context affect clinical trials in Colombia?
Understanding local healthcare practices and patient preferences is crucial for improving recruitment strategies and study design. Tailoring approaches to cultural nuances can significantly enhance participant engagement and retention in clinical trials.
What economic factors should be considered when building trial partnerships in Colombia?
Evaluating the economic landscape, including funding opportunities and partnerships with local stakeholders, is critical for efficient trial execution. The demand for innovative medical devices and the country's developed healthcare infrastructure create a favorable environment for medical research.
What services does Bioaccess provide for managing studies in Colombia?
Bioaccess offers extensive management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, which are vital for navigating the complexities of research studies in Colombia.
What is the role of INVIMA in Colombia's Medtech landscape?
The Colombian National Food and Drug Surveillance Institute (INVIMA) regulates medical devices and ensures compliance with health standards. As a Level 4 health authority recognized by PAHO/WHO, INVIMA's oversight is crucial for maintaining the integrity of medical research in the country.
What steps are essential for regulatory compliance in Colombia?
Key steps include understanding INVIMA requirements, preparing necessary documentation, engaging with local ethics committees, monitoring regulatory changes, and utilizing local expertise, such as partnering with contract research organizations like bioaccess.
What are the benefits of conducting clinical studies in Colombia?
Colombia is the sixth-largest contributor to research studies in Latin America, offering generous R&D tax incentives, including a 50% tax rebate on research investments. The regulatory process has also improved, with inter-trial intervals reduced to 17 months as of 2025.




