Overview
The primary objective of this article is to deliver a comprehensive understanding of the regulatory pathways for medical technology (Medtech) in Colombia. It underscores the importance of navigating these pathways, particularly through the guidance of INVIMA and adherence to Decree 4725 of 2005. This navigation is essential for ensuring compliance and facilitating market entry for Medtech companies. The detailed stages of the regulatory process illustrate the significant growth of the sector, driven by these frameworks, highlighting the collaborative efforts necessary for success.
Introduction
Colombia's Medtech sector is experiencing significant growth, propelled by an increasing demand for innovative medical devices and an evolving regulatory landscape designed to meet this need.
Central to this framework is Decree 4725 of 2005, which sets forth stringent guidelines for the registration and oversight of medical devices, ensuring that safety and efficacy are prioritized.
As companies navigate this complex terrain, the role of the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) becomes indispensable, acting as the gatekeeper for market entry.
With projections indicating substantial expansion in the Colombian market, a deep understanding of regulatory compliance intricacies is essential for Medtech firms seeking to seize new opportunities.
This article explores the regulatory processes, challenges, and best practices that characterize the Medtech landscape in Colombia, offering valuable insights for companies aiming to excel in this dynamic environment.
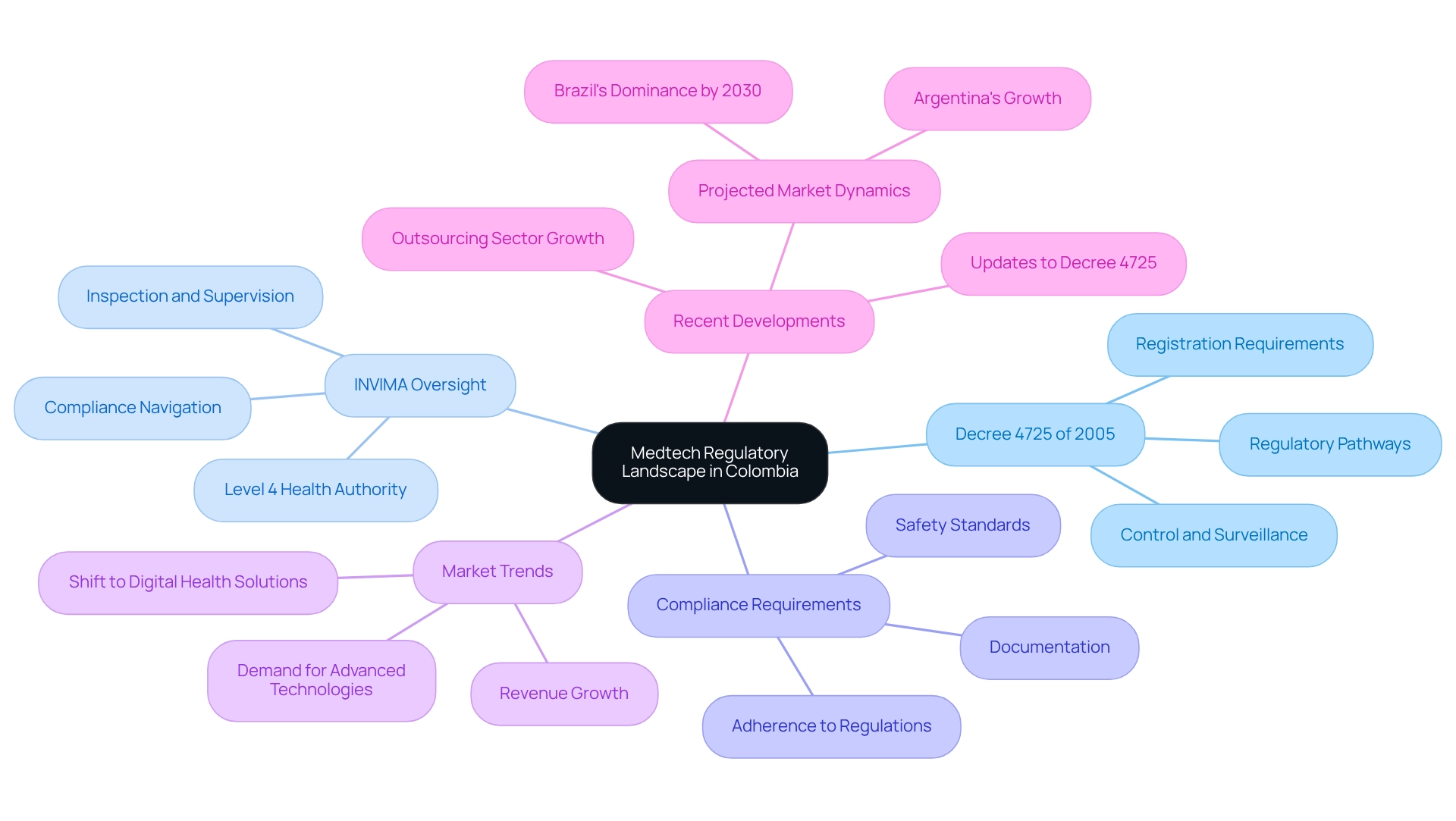
Overview of Medtech Regulatory Landscape in Colombia
The Medtech environment in Colombia is shaped by robust regulatory pathways designed to ensure the safety and effectiveness of medical devices. Central to this framework is Decree 4725 of 2005, which delineates the regulatory pathways for medtech, detailing the essential requirements for the registration, control, and surveillance of medical devices. This decree is pivotal in guiding companies through the compliance process, making it imperative for Medtech firms to possess a thorough understanding of these regulatory pathways.
The Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) functions as the regulatory authority overseeing these pathways. Classified as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA is tasked with inspecting and supervising the marketing and manufacturing of health products. Companies aspiring to enter the industry must adeptly navigate INVIMA's requirements, which necessitate a comprehensive understanding of the regulatory pathways, meticulous documentation, and strict adherence to safety standards.
Grasping the regulatory pathways for medtech in Colombia is crucial for ensuring compliance and facilitating a smoother entry into the industry.
Recent developments reveal that the Colombian Medtech sector is witnessing significant growth, largely propelled by these regulatory pathways and the escalating demand for advanced medical technologies. In 2024, the outsourcing sector emerged as the leading revenue-generating service provider in Colombia, indicative of a trend towards collaboration with specialized firms to efficiently meet compliance demands. As noted by MarkWide Research, "Their reports are thorough, accurate, and delivered on time. We value their professionalism and expertise, and would strongly endorse their services to other companies seeking dependable research."
Moreover, the oversight landscape is evolving, with updates to Decree 4725 of 2005 aimed at streamlining processes and enhancing the approval timeline for innovative devices. This evolution is essential as Brazil is projected to dominate the medical device Regulatory Affairs sector in Latin America by 2030, while Argentina is anticipated to be the fastest-growing regional sector, reaching USD 67.9 million by the same year.
The Medical Technology sector in Colombia has been undergoing substantial expansion, driven by consumer preferences for advanced medical devices, favorable local conditions, and macroeconomic factors. The demand for innovative medical technology has surged among patients and healthcare providers, resulting in the introduction of cutting-edge medical devices. Successful Medtech companies in Colombia have demonstrated their capacity to effectively navigate the regulatory pathways, leveraging their understanding of the local landscape to bring innovative solutions to market.
Bioaccess provides a comprehensive range of services to enhance medical device evaluations, including:
- Feasibility studies
- Selection of research locations
- Principal investigator (PI) identification
They offer thorough reviews and feedback on study documents to ensure compliance with local requirements, manage trial set-up and start-up processes, and facilitate the import permit and nationalization of investigational devices. As the demand for digital health solutions and minimally invasive surgical procedures rises, the regulatory pathways for medtech in Colombia, coupled with supportive government policies and investments in healthcare infrastructure, underscore the significance of compliance.
Businesses that align their strategies with the evolving legal framework will be better positioned to capitalize on the burgeoning opportunities within Colombia's Medtech sector.
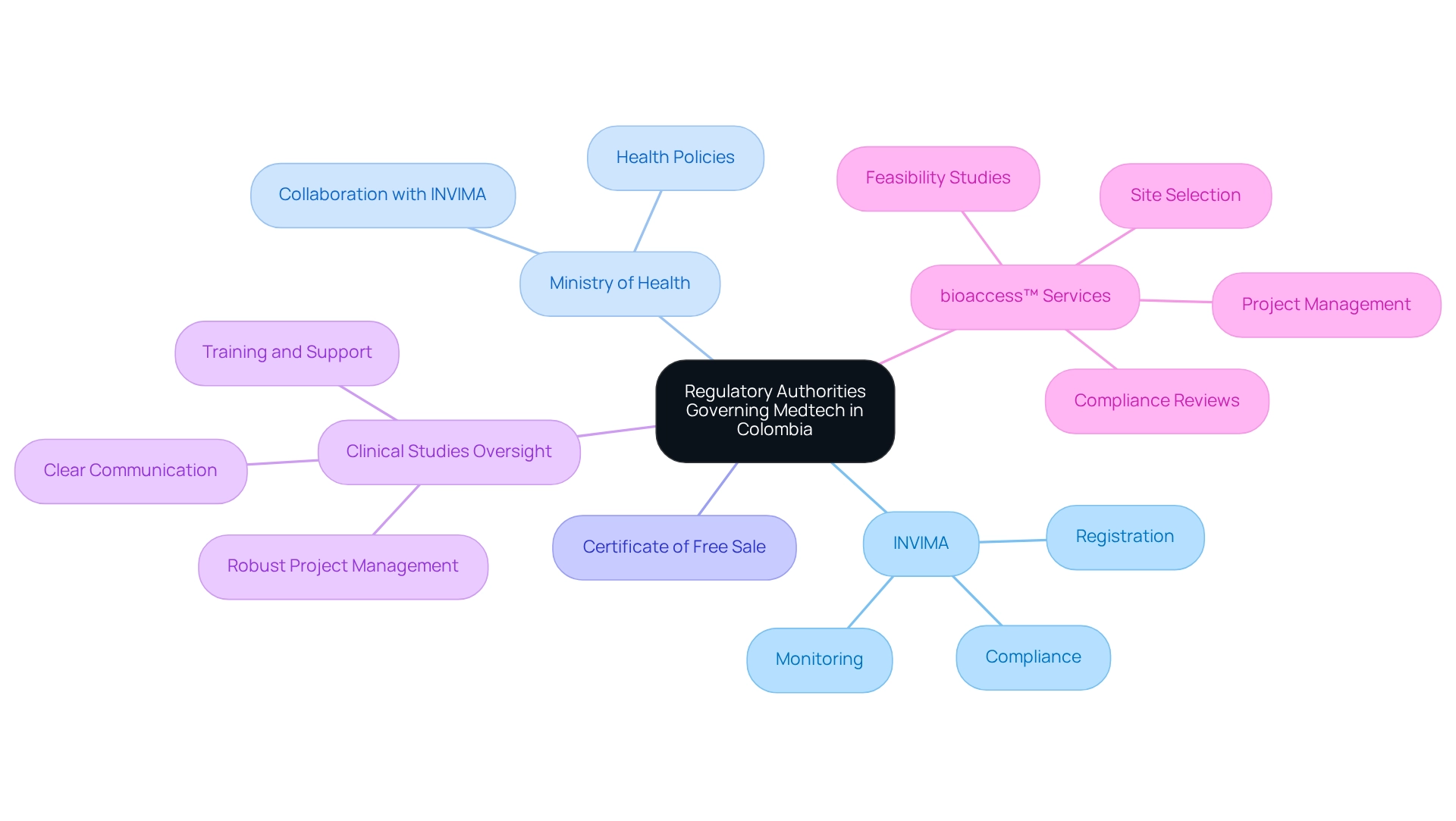
Key Regulatory Authorities Governing Medtech in Colombia
In Colombia, the regulatory pathways for medtech are overseen by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which is responsible for the registration and monitoring of medical devices. INVIMA plays a crucial role in evaluating the safety and efficacy of products prior to their market introduction, ensuring that only compliant devices reach healthcare providers and patients. As of 2025, INVIMA has streamlined its processes, resulting in a notable increase in the number of medical device approvals. This reflects a commitment to fostering innovation while maintaining high safety standards.
The Ministry of Health significantly influences the Medtech landscape by establishing health policies and regulations that govern the sector. This ministry collaborates closely with INVIMA to ensure that oversight frameworks align with public health objectives. Companies seeking to navigate the regulatory pathways for medtech in Colombia must engage with both INVIMA and the Ministry of Health throughout the product lifecycle, from initial registration to post-market surveillance.
Notably, the Certificate of Free Sale is a requirement for the registration of medical instruments with INVIMA, verifying the device's approval for sale in its country of origin and underscoring its significance in the regulatory pathways for medtech in Colombia.
Optimal approaches for overseeing medical device clinical studies in Colombia highlight the significance of clear communication among stakeholders, robust project management strategies, and thorough training and assistance for all participants. Consistent oversight and evaluation, combined with the application of cutting-edge technology for data handling, are crucial for upholding compliance and ensuring the integrity of clinical studies. bioaccess™ provides a variety of service capabilities, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, which are essential for successful execution.
A case analysis on optimal methods for medical device testing in the country emphasizes the importance of efficient communication tactics and local stakeholder involvement. This study illustrates how researchers can enhance study design, improve participant recruitment and retention, and ultimately contribute to the advancement of healthcare solutions tailored to the needs of the population. For instance, bioaccess™ has partnered with Welwaze Medical Inc. to effectively introduce the Celbrea® medical device in Colombia, showcasing the efficacy of strategic collaborations in navigating compliance processes and achieving substantial results, such as enhanced access and adherence.
As highlighted by industry specialists, 'Clear objectives are essential to the success of clinical trials.' This underscores the need for Medtech companies to understand the regulatory pathways for medtech in Colombia when establishing well-defined goals while engaging with oversight bodies. This proactive strategy not only enables smoother approvals but also aligns product development with compliance expectations, paving the way for successful entry by navigating the regulatory pathways for medtech in Colombia. With over 20 years of experience in the Medtech sector, bioaccess™ is well-equipped to assist companies through these compliance pathways.
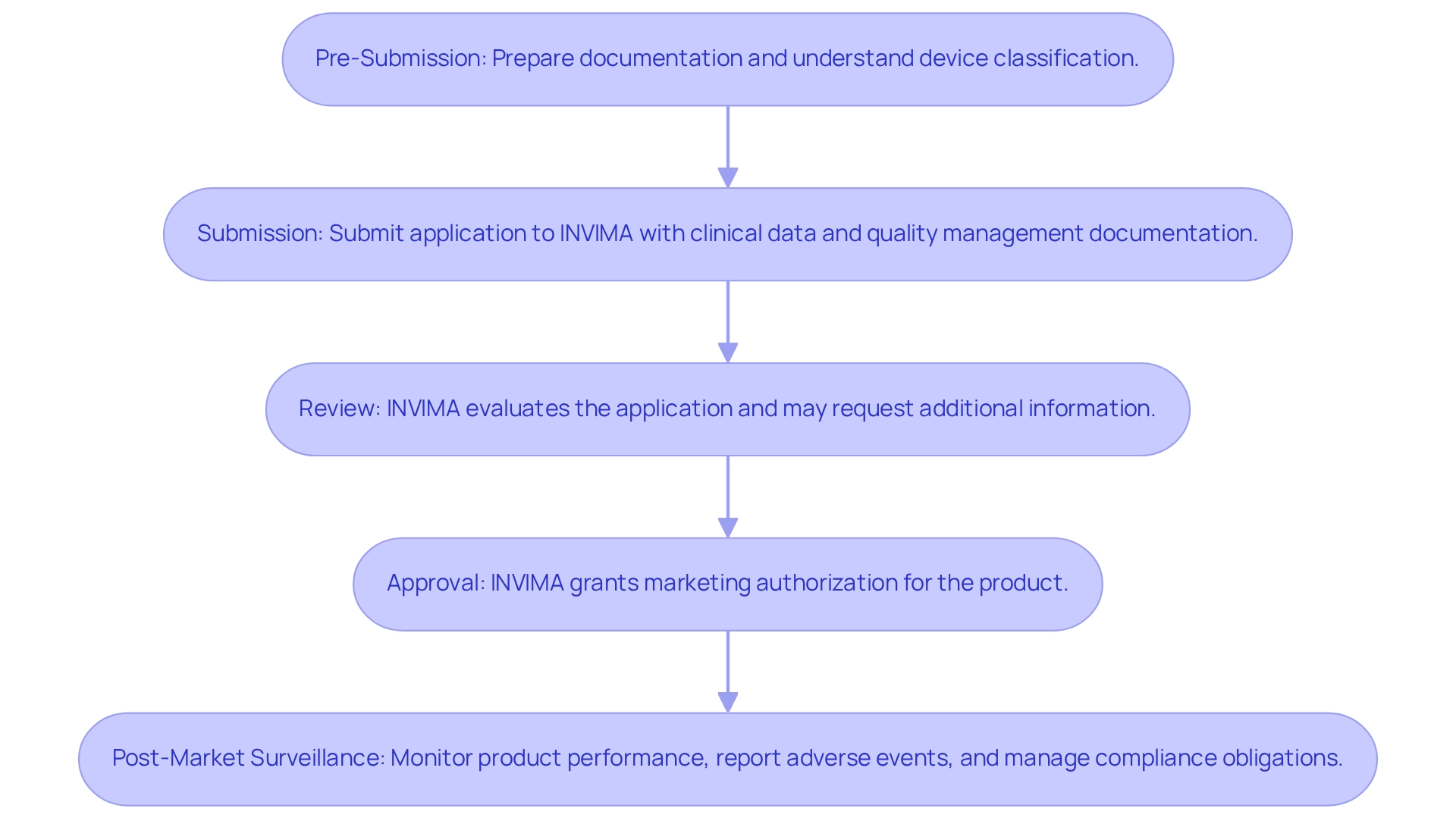
Stages of the Regulatory Process for Medtech Products
The oversight procedure for Medtech products in Colombia is structured around regulatory pathways, encompassing several essential phases that guarantee adherence and facilitate access to the sector. These stages are as follows:
-
Pre-Submission: In this initial phase, companies prepare the necessary documentation, including a thorough understanding of device classification, which is crucial for determining the compliance pathway. This preparation is vital for navigating the Latin American Medtech landscape efficiently, particularly regarding regulatory pathways in Colombia, as it lays the groundwork for successful entry.
-
Submission: A comprehensive application is submitted to INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos). This application must include detailed clinical data, quality management system documentation, and any other relevant information that supports the safety and efficacy of the device. Firms such as bioaccess® play a crucial role in assisting clients with this process, ensuring that submissions comply with all guidelines and are tailored to the specific demands of the Colombian sector.
-
Review: INVIMA conducts a meticulous evaluation of the submitted application. This stage may involve additional queries or requests for further information, emphasizing the importance of thorough and accurate submissions to avoid delays. The expertise of organizations like bioaccess® significantly enhances submission quality, thereby improving the chances of timely approval.
-
Approval: Upon successful review, INVIMA grants marketing authorization, allowing the product to enter Colombia. This stage is critical, as it signifies that the product meets all regulatory requirements. The successful navigation of regulatory pathways by companies such as Mitralign and Cook Medical illustrates the potential for growth within this industry, showcasing how effective strategies can lead to successful entry.
-
Post-Market Surveillance: After approval, companies are required to actively monitor the product's performance in the market. This includes reporting any adverse events to INVIMA, which is essential for maintaining compliance and ensuring ongoing safety for users. Furthermore, companies must manage renewals, modifications, and vigilance requirements to uphold their compliance obligations. bioaccess® is committed to ensuring information security and client trust throughout this process, addressing any concerns related to data protection. For more detailed guidance, clients can refer to bioaccess®'s user manuals, which outline compliance processes and provide insights into grievance procedures to ensure adherence and transparency.
Statistics indicate that Colombia stands as Latin America's third-largest medical device market, following Brazil and Mexico, underscoring the importance of successfully navigating the regulatory pathways for Medtech in Colombia. However, challenges persist, as demonstrated by a case study on compliance that uncovered weak enforcement of study registration and result reporting, raising ethical concerns. This highlights the necessity for ethical compliance in research, with studies showing that adherence to these standards can improve Phase I success rates to 48% and Phase III rates to 66%.
As ethicist Jennifer Miller aptly states, "Ethics is not minimal compliance with the law," reinforcing the importance of a robust ethical framework in the oversight process.
As the governing environment evolves, understanding the regulatory pathways for Medtech in Colombia regarding product submission to INVIMA is paramount. Experts stress that ethical considerations extend beyond simple adherence to the law, highlighting the necessity for a strong method in oversight processes within the Medtech sector, a principle that bioaccess® maintains in its operations.
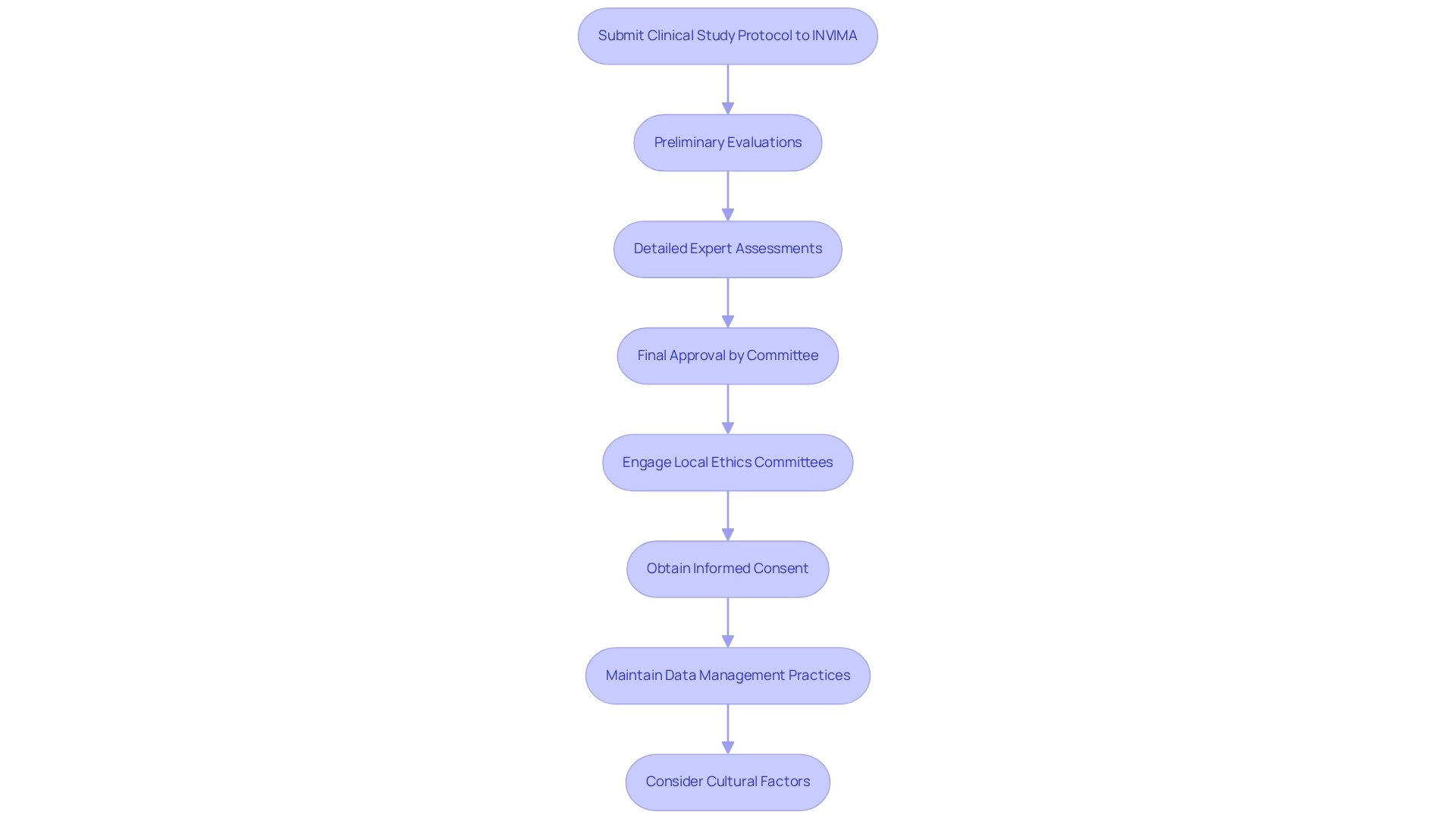
Clinical Trial Requirements and Best Practices in Colombia
Conducting clinical studies in Colombia necessitates strict adherence to the regulatory pathways for medtech, alongside established best practices. Companies must present a thorough clinical study protocol to INVIMA, outlining objectives, methodologies, and ethical considerations. This protocol undergoes a rigorous review process, including preliminary evaluations, detailed expert assessments, and final approval by a higher-level committee, ensuring that all studies meet the necessary safety and scientific validity standards.
Optimal methods for clinical studies in Colombia highlight the significance of early involvement with local ethics committees. This proactive approach facilitates smoother approvals and fosters trust within the community. Furthermore, acquiring informed consent from participants is crucial, guaranteeing that they are fully aware of the study's nature and possible risks.
Data management practices must be meticulously maintained to uphold the integrity of the experiment. Companies should also be mindful of cultural factors and language barriers when creating study materials, as these elements significantly influence participant engagement and retention.
The legal framework in the country is especially conducive for medical device studies, featuring a classification system for in vitro diagnostic (IVD) devices categorized into three classes: I, II, and III. This categorization assists in simplifying the approval procedure, making the country an appealing location for U.S. medical device firms aiming to carry out clinical studies. The nation's blend of a significant population, skilled research facilities, and effective oversight procedures has resulted in a history of successful clinical studies, further reinforcing its status as a major player in the Medtech industry.
As Julio G. Martinez-Clark, CEO of bioaccess, observed, "The government of Colombia appears to be the sole nation in Latin America with a proactive effort to draw more clinical studies as part of its strategy to transform into a knowledge economy by 2031." This commitment highlights the encouraging framework for clinical studies.
Moreover, INVIMA has specific requirements for approving first-in-human medical device studies, emphasizing safety, scientific validity, and ethical conduct. By adhering to the regulatory pathways for medtech in Colombia, companies can effectively manage the complexities of clinical studies in the country, ultimately contributing to the advancement of medical technologies that enhance patient care. bioaccess® stands out as a leading contract research organization facilitating these clinical trials, ensuring compliance and efficiency throughout the process, including import permits and nationalization of investigational devices, as well as comprehensive reporting on study status, inventory, and adverse events.
Navigating Local Regulations: Strategies for Medtech Companies
Navigating local regulations in Colombia necessitates a thorough understanding of the regulatory pathways for medtech, compelling Medtech companies to implement several essential strategies.
- Engage Local Experts: Collaborating with regulatory consultants who possess a profound understanding of the Colombian regulatory landscape is vital. These local experts provide insights tailored to the specific nuances of the market, ensuring compliance and operational efficiency.
bioaccess™ is dedicated to advancing medical devices more swiftly through its expertise in comprehensive clinical trial management services, which encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. This positions bioaccess™ as a valuable partner in this intricate process.
-
Stay Informed: Consistently examining updates from INVIMA, the Colombia National Food and Drug Surveillance Institute, and other oversight organizations is crucial. Staying informed about regulatory changes enables companies to adjust swiftly and maintain compliance, which is essential in a dynamic legal environment. For example, awareness that the timeline for a lawsuit against ABIMED for GMP inspection can extend to 6-8 months underscores the importance of timely compliance. bioaccess™ aids clients in navigating these updates by providing tailored guidance and support.
-
Build Relationships: Establishing robust communication channels with governing bodies can facilitate smoother interactions. Developing connections with these entities not only aids in maneuvering through the compliance process but also fosters trust, which can be advantageous during inspections or audits. Collaborations, such as those between bioaccess™ and Caribbean Health Group, position Barranquilla as a prominent destination for clinical trials in Latin America, supported by the nation's Minister of Health.
-
Tailor Documentation: All submissions must be meticulously tailored to meet local requirements, including language and formatting. This attention to detail can significantly influence the approval process, as oversight organizations expect documentation that aligns with their standards. bioaccess™ ensures that all documentation is prepared in accordance with INVIMA's specific requirements, thereby increasing the likelihood of approval.
The outsourcing sector in Colombia's medical device compliance field has emerged as a substantial revenue generator, indicating a trend towards leveraging local expertise. In 2024, this segment was identified as the largest service provider, with projections indicating rapid growth. Involving local compliance advisors not only enhances adherence but also enables Medtech firms to effectively navigate the regulatory pathways for medtech in Colombia and capitalize on lucrative opportunities.
Successful collaborations, such as Welwaze Medical Inc.'s alliance with bioaccess™ for the Celbrea® medical device launch, can lead to expedited approvals and a more streamlined route to commercialization, ultimately advancing medical devices more effectively. Moreover, events like the Foreign Trade Day for the Pharma and Medtech Industry 2025 in Berlin offer valuable networking opportunities that can further bolster these strategies.
Challenges in the Regulatory Pathway for Medtech in Colombia
Medtech firms operating in Colombia face a myriad of challenges when navigating the regulatory pathways, significantly impacting their operations and timelines. Key issues include:
-
Complex Documentation Requirements: The extensive documentation mandated for medical devices often leads to considerable delays in the approval process. Businesses frequently find themselves overwhelmed by the volume and detail of the required documentation, which can hinder timely entry into the industry.
-
Lengthy Approval Times: Despite notable improvements in efficiency by INVIMA, Colombia's governing body, approval times for certain products remain prolonged. This situation can be particularly challenging for startups and smaller companies that depend on rapid market access to sustain their operations and growth.
-
Cultural and Language Barriers: Navigating local language requirements and cultural nuances presents additional hurdles for foreign companies. Effective communication is essential, as misunderstandings can result in misinterpretations of regulatory expectations, complicating the approval process further. Cultural similarities among Costa Rica, the U.S., and other nations—such as work ethic and adaptability—enhance the integration of nearshore software teams with American businesses, providing a competitive advantage for Medtech companies.
-
Regulatory Changes: The regulatory landscape is dynamic, with frequent updates that create uncertainty for Medtech companies. Staying informed about these changes is crucial, as companies must adapt swiftly to remain compliant and avoid potential setbacks.
These challenges extend beyond mere bureaucratic hurdles; they can have tangible consequences for the success of regulatory pathways for medtech in Colombia. For instance, a case study highlighting the impact of clinical studies on local economies illustrates how compliance obstacles can affect not only the healthcare sector but also broader economic growth. Agreements like MERCOSUR facilitate trade and collaboration, yet the complexities of documentation and approval processes can impede progress.
As noted by Julio G. Martinez-Clark, CEO of bioaccess Colombia, "This nation's combination of a large and diverse population, experienced clinical research sites, efficient regulatory processes, a cost-competitive environment, and a track record of successful medtech clinical trials since 2010 make it an appealing option for U.S. medical device companies seeking to conduct clinical research."
Moreover, the statistic indicating that 37% of respondents expressed interest in a medication and lab test discount membership underscores the demand for Medtech innovations in Colombia. Expert insights suggest that a comprehensive approach—integrating compliance, industry understanding, and collaboration—is essential for success in the Latin American Medtech sector. Addressing these documentation challenges requires strategic planning and a proactive compliance engagement approach, particularly concerning the regulatory pathways for medtech in Colombia, ensuring that companies can effectively navigate the complexities of the Colombian market.
The Role of CROs in Supporting Medtech Regulatory Compliance
Contract research organizations (CROs) serve as essential collaborators for Medtech companies navigating the regulatory pathways in Colombia. Their specialized expertise in clinical study design, compliance submissions, and post-market monitoring significantly enhances the efficiency of the approval process. By ensuring that all documentation adheres to the standards set by INVIMA, the governing body, CROs play a critical role in expediting approvals.
Leveraging their local knowledge, CROs provide insights that improve study implementation, aiding companies in adapting to the unique compliance landscape. By managing relationships with regulatory bodies, they facilitate smoother communication, which can lead to faster approvals and a reduced time to market. Colombia's substantial population and effective regulatory framework further bolster its attractiveness for U.S. Medtech firms, positioning it as an ideal site for clinical studies.
The impact of CROs on the success of clinical studies in Colombia is evident in numerous successful investigations conducted by various companies, including ReGelTec, which recently completed an Early Feasibility Study for HYDRAFIL™ aimed at addressing chronic low back pain. This research, conducted in Barranquilla, underscored the potential for Medtech advancements in the region, supported by the collaboration between bioaccess and Caribbean Health Group to establish Barranquilla as a premier location for clinical studies in Latin America.
Statistics indicate that MCRA oversees over 1,700 projects annually, underscoring the pivotal role CROs play in the Medtech industry and their influence on achieving successful clinical outcomes. GlobalCare Clinical Studies, in partnership with bioaccess, has achieved more than a 50% reduction in recruitment time and maintained 95% retention rates, further illustrating the effectiveness of CROs in enhancing clinical study ambulatory services in Colombia.
As the landscape evolves in 2025, insights from CRO professionals will continue to be vital in optimizing the approval process, ensuring that innovative medical devices can enter the market efficiently and safely. As Julio G. Martinez-Clark emphasized, a Medical Device CRO is indispensable for conducting clinical trials in the country due to its expertise in regulatory pathways, access to quality healthcare infrastructure, cost-effectiveness, and capacity to assure the quality and safety of clinical trials. The collaboration between Medtech companies and CROs not only enhances compliance but also fosters a thriving environment for clinical research in the region.
Testimonials from industry leaders, such as Bill Andrews, Ph.D., President & CEO of Sierra Sciences, further highlight the positive impact of these collaborations.
Best Practices for Successful Regulatory Navigation in Medtech
To successfully navigate the regulatory landscape in Colombia, Medtech companies must implement several best practices:
-
Early Engagement: Initiating discussions with INVIMA (Colombia National Food and Drug Surveillance Institute) at the outset of the product development process is crucial. Engaging early can significantly streamline the approval process, as numerous case studies demonstrate that proactive communication leads to faster approvals and reduced time to market. Statistics indicate that companies prioritizing early engagement experience a higher rate of successful approvals.
-
Thorough Documentation: Preparing comprehensive and well-organized documentation for submissions is essential. This not only facilitates a smoother review process but also reflects the company’s commitment to compliance with regulations, positively influencing INVIMA's assessment. The Ministry of Finance's 2021 roadmap for modernizing the insurance sector highlights the need for convergence with IFRS 17 standards, underscoring the importance of adherence to standards in the Medtech context.
-
Continuous Training: Investing in ongoing training for staff on compliance requirements and best practices is vital. Keeping the team informed about the latest rules and compliance strategies ensures that the organization remains agile and responsive to changes in the legal landscape.
-
Feedback Loops: Establishing systems for obtaining feedback from oversight authorities can enhance future submissions. By actively seeking input from INVIMA, companies can refine their approaches and improve the quality of their documentation, ultimately leading to more successful outcomes.
-
Automation and Data Integrity: As illustrated in the case study titled "Embracing Automation and Strengthening Data Integrity," investing in automation and data lineage is essential for optimizing operational processes and ensuring adherence to reporting requirements. Automating key processes, supported by machine learning and AI, strengthens control frameworks, while data lineage tools ensure data integrity and compliance throughout its lifecycle.
The significance of early involvement with INVIMA cannot be overstated; as the compliance environment evolves, particularly with the new administration potentially overturning previous executive orders related to mergers and climate risks, understanding the regulatory pathways for Medtech in Colombia and remaining adaptable is crucial for companies striving to succeed in this dynamic market. As Harris Stevenson-Robb, Manager, noted, "Al enviar este formulario, entiendo que mi información será procesada por Capgemini como se indica arriba, y como se describe en los Política de Protección de Datos," emphasizing the critical nature of comprehending regulatory processes and compliance.
Conclusion
The Medtech sector in Colombia is experiencing significant growth, propelled by a strong regulatory framework and an increasing demand for advanced medical devices. Central to this is Decree 4725 of 2005, which ensures the safety and efficacy of these products, while INVIMA acts as the pivotal regulatory authority overseeing the approval process. A comprehensive understanding of this regulatory landscape is crucial for companies seeking to excel in this dynamic market.
Navigating the regulatory pathway encompasses multiple stages, from pre-submission preparations to post-market surveillance. Companies must be adept in local requirements and actively engage with regulatory authorities to facilitate smoother interactions. The role of Contract Research Organizations (CROs) is vital, as these entities offer essential support in managing compliance and expediting the approval process.
Despite the promising growth potential, Medtech companies encounter challenges such as intricate documentation requirements and prolonged approval times. Overcoming these obstacles necessitates strategic planning and proactive engagement with INVIMA. By adopting best practices—including thorough documentation, early engagement, and ongoing training—companies can significantly improve their prospects for successful market entry.
Ultimately, success in Colombia's Medtech sector depends on a deep understanding of the regulatory framework and a steadfast commitment to compliance. As the market evolves, companies that align their strategies with these regulatory demands will be optimally positioned to seize emerging opportunities, fostering innovation and enhancing healthcare outcomes in the region.
Frequently Asked Questions
What is the primary regulatory framework for medical devices in Colombia?
The primary regulatory framework for medical devices in Colombia is outlined in Decree 4725 of 2005, which specifies the regulatory pathways for registration, control, and surveillance of medical devices.
Who oversees the regulatory pathways for medtech in Colombia?
The regulatory pathways for medtech in Colombia are overseen by the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA), which is responsible for inspecting and supervising the marketing and manufacturing of health products.
What is the role of INVIMA in the medtech sector?
INVIMA evaluates the safety and efficacy of medical devices before they are introduced to the market, ensuring that only compliant products are available to healthcare providers and patients.
How has INVIMA's process for medical device approvals changed recently?
As of 2025, INVIMA has streamlined its processes, leading to a notable increase in the number of medical device approvals, reflecting a commitment to fostering innovation while maintaining high safety standards.
What is the significance of the Certificate of Free Sale in the registration process?
The Certificate of Free Sale is required for registering medical instruments with INVIMA, as it verifies that the device has been approved for sale in its country of origin.
What services does Bioaccess provide to support medical device evaluations?
Bioaccess provides services including feasibility studies, selection of research locations, principal investigator identification, compliance reviews, trial setup, import permits, project management, and reporting.
What factors are driving growth in Colombia's Medtech sector?
Growth in Colombia's Medtech sector is driven by consumer preferences for advanced medical devices, favorable local conditions, macroeconomic factors, and an increasing demand for innovative medical technologies.
What strategies are essential for overseeing medical device clinical studies in Colombia?
Effective communication among stakeholders, robust project management strategies, and thorough training for participants are essential for overseeing medical device clinical studies in Colombia.
How can Medtech companies align their strategies with the evolving regulatory landscape?
Medtech companies can align their strategies with the evolving regulatory landscape by understanding the regulatory pathways and engaging with INVIMA and the Ministry of Health throughout the product lifecycle.
What collaborative efforts have been noted in the introduction of medical devices in Colombia?
Strategic collaborations, such as the partnership between Bioaccess and Welwaze Medical Inc. for the introduction of the Celbrea® medical device, showcase the effectiveness of working together to navigate compliance processes and achieve substantial results.

