Overview
The main considerations for medtech companies entering Latin America include navigating regulatory compliance, understanding local market dynamics, and establishing strategic partnerships. The article emphasizes that successful market entry hinges on thorough knowledge of regional regulations, effective distribution channels, and cultural sensitivities, all of which are crucial for ensuring product acceptance and adherence to compliance standards.
Introduction
Entering the Latin American market presents a unique set of opportunities and challenges, particularly in the medtech sector. With a diverse regulatory landscape and varying consumer preferences across countries, companies must adopt a strategic approach to ensure successful market penetration. Key factors such as:
- Regulatory compliance
- Thorough market research
- The establishment of strong distribution channels
are essential for navigating this complex environment.
Furthermore, understanding local cultural nuances and engaging in strategic partnerships can significantly enhance a company's ability to connect with healthcare providers and consumers alike. As the region continues to evolve, a comprehensive understanding of these dynamics will be crucial for organizations aiming to thrive in the burgeoning Latin American medtech market.
Key Factors for Successful Market Entry in Latin America
Achieving successful entry in South America necessitates a strategic approach that encompasses several key factors:
-
Regulatory Compliance: Navigating the intricate landscape of regional regulations is essential. Each Latin American nation has unique regulations governing medical devices, and failure to comply can lead to significant obstacles, including delays or outright exclusion. To mitigate risks, Top Considerations for Medtech Companies Entering Latin America include being proactive in understanding these regulations given the current environment. Notably, of the 43 companies that reached FCPA-related resolutions between 2019 and 2023, the US government pursued at least 42 individuals related to the conduct of at least 10 companies, underscoring the critical importance of compliance in the context of anti-corruption efforts.
-
Industry Analysis: Comprehensive industry analysis is essential for understanding regional healthcare requirements, competitive dynamics, and suitable pricing strategies. A comprehensive understanding of these elements is among the top considerations for medtech companies entering Latin America, as it facilitates effective product positioning and enhances the likelihood of acceptance among consumers and healthcare providers.
- Distribution Channels: Establishing the right distribution partnerships is crucial for gaining access to healthcare providers and end-users. Effective distribution strategies are one of the top considerations for medtech companies entering Latin America, as they can significantly impact the reach and success of medical devices in the market. Developing a competitive pricing strategy that corresponds with regional economic conditions and consumer purchasing power is one of the top considerations for medtech companies entering Latin America. A well-considered approach to pricing can differentiate a product in a crowded marketplace.
-
Regional Knowledge: Working with regional specialists and advisors can offer invaluable perspectives on industry dynamics and regulatory requirements. Their knowledge is one of the top considerations for medtech companies entering Latin America, as it can enhance a company's strategy, leading to improved chances of success and smoother adherence to regional laws. In the context of recent trends, one of the top considerations for medtech companies entering Latin America is to prioritize strong anti-corruption policies, as highlighted by the ongoing challenges in the region. The Northern Triangle Enhanced Engagement Act underscores the necessity for robust compliance measures in light of systemic corruption, which has seen several former government officials face trials related to bribery and money laundering. As noted by Nicole M Argentieri, the Acting Assistant Attorney, 'corporate criminal enforcement and individual accountability are two sides of the same coin,' emphasizing the importance of self-disclosure and cooperation to build stronger compliance frameworks.
Additionally, the comprehensive process for advancing medical device trials in Colombia includes feasibility and selection of research sites, principal investigator (PI) selection, trial set-up, start-up, and approval through INVIMA approvals and ethics committee reviews, as well as study project management and monitoring. Companies such as Welwaze Medical Inc. and Hancock Jaffe Laboratories have effectively navigated this landscape, utilizing regional expertise through consulting firms like bioaccess™ to facilitate their clinical trials and access initiatives. Bioaccess™ played a crucial role in guiding these companies through compliance and project management, ensuring a smoother entry into the Colombian market. These collaborations highlight the top considerations for medtech companies entering Latin America, showcasing Colombia's competitive advantages such as cost efficiency, swift processes, high-quality healthcare, and incentives for research and development, making it an appealing location for first-in-human studies.
BOOK A MEETING to discuss how we can assist you in advancing your medical device trials in South America.
Navigating Regulatory Landscapes and Healthcare Systems
Navigating the compliance environment in South America requires a strategic approach that encompasses several critical factors:
- Understanding Local Regulations: Each country in Latin America has its own regulatory authority, such as ANVISA in Brazil and COFEPRIS in Mexico, alongside INVIMA in Colombia, which is recognized as a Level 4 health authority by PAHO/WHO. These agencies impose specific requirements for medical devices, and understanding these regulations is essential for compliance.
- Comprehensive Clinical Trials: Conducting regional clinical trials is often vital for demonstrating the safety and efficacy of medical devices. This process necessitates a thorough understanding of regional ethical standards and protocols, which can vary significantly from one country to another. As Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, highlights, local insights can greatly enhance compliance efforts and the overall success of clinical studies. Our service capabilities include feasibility studies and site selection to ensure optimal trial locations and principal investigator (PI) selection.
- Approval Processes: Familiarity with the approval processes is crucial. Companies must navigate the documentation requirements and timelines specific to each governing body to avoid costly delays in product launches. Notably, the transition to online submissions for medical device registrations is being implemented by several regulatory agencies, moving away from paper-based procedures. This shift is expected to enhance the efficiency of the registration process, although the pace of implementation varies by country. Our services also cover compliance reviews to ensure all study documents align with local requirements.
- Healthcare System Structure: An in-depth understanding of the healthcare system's structure—including both public and private sectors—within each country can inform effective market strategies and facilitate successful product placements. This knowledge is especially relevant as South America experiences a shift towards online submissions for medical device registrations.
- Effect on Nearby Economies: Participating in clinical trials not only assists in compliance with regulations but also supports nearby economies through job creation and healthcare advancements. The MERCOSUR agreement, which has eliminated tariffs on about 90% of goods traded between member states, further facilitates trade and collaboration in the region. Furthermore, our project management services guarantee that studies are monitored efficiently, and reporting on study status, inventory, and adverse events is performed comprehensively.
By taking these aspects into account and working with regional specialists, organizations can more effectively navigate the complex regulatory environment of Central and South America, which are among the top considerations for medtech companies entering Latin America, positioning themselves for successful entry and sustained growth in the medtech sector.
The Role of Partnerships in Medtech Success
One of the top considerations for medtech companies entering Latin America is the importance of strategic partnerships for attaining success in the sector. Here are key avenues through which collaboration can yield significant benefits:
-
Regional Distributors: Partnering with established regional distributors offers prompt access to a well-entrenched network and customer base, essential in a diverse environment characterized by varying regulations and consumer preferences.
Their expertise can facilitate smoother market entry and reduce potential hurdles.
-
Healthcare Providers: Partnerships with hospitals and clinics not only allow for product testing and validation but also foster trust with end-users. This collaboration is essential as healthcare providers can offer valuable insights into community needs and preferences, ultimately enhancing product acceptance.
-
Research Organizations: Partnering with nearby research organizations is essential for maneuvering through the intricate compliance environment and performing required clinical studies, such as those observed by Dushyanth Surakanti during the initial human trial of bioaccess® in Colombia. These partnerships enhance the credibility of research efforts and ensure compliance with local standards, thereby facilitating smoother project execution.
-
Government Agencies: Engaging with government bodies, including INVIMA, the Colombia National Food and Drug Surveillance Institute, is vital for understanding evolving policy landscapes and securing necessary approvals.
This collaboration reduces risks related to compliance and assists in navigating potential challenges in the market.
-
Comprehensive Clinical Trial Management Services: Partnering with organizations that offer comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—can significantly enhance the efficiency and effectiveness of clinical research initiatives. These services ensure that all aspects of the trial are managed professionally, leading to better outcomes and adherence to regulatory standards.
The trade balance of health equipment in the region of America and the Caribbean has reached an impressive 10 billion USD from 2018 to 2023, highlighting the immense potential for growth in this sector. This statistic underscores the top considerations for medtech companies entering Latin America, highlighting the importance of strategic partnerships as companies look to capitalize on this growth. As Jennifer Mendoza, a research expert in health and medtech, emphasizes,
Statista assumes no liability for the information given being complete or correct,
which points to the necessity of thorough due diligence in partnerships.
Furthermore, it is essential to recognize the ethical challenges in conducting clinical research abroad, such as those faced during Avinger's OCT-guided atherectomy research in Cali, Colombia. These challenges can significantly impact the success of medtech initiatives, emphasizing the need for ethical considerations and local engagement. Cultural exchange plays a fundamental role in enhancing mutual understanding among stakeholders, which can lead to innovative solutions and improved patient care.
By fostering open dialogue and respect, stakeholders can build stronger connections that are essential for navigating the complexities of the American medtech landscape.
Understanding Cultural Nuances and Consumer Preferences
Understanding cultural nuances and consumer preferences are among the top considerations for medtech companies entering Latin America, particularly within the regulatory landscape defined by authorities such as INVIMA, which oversees the safety and quality of medical devices. Understanding these elements is essential for companies, particularly in the context of the top considerations for medtech companies entering Latin America, to thrive in the medtech sector.
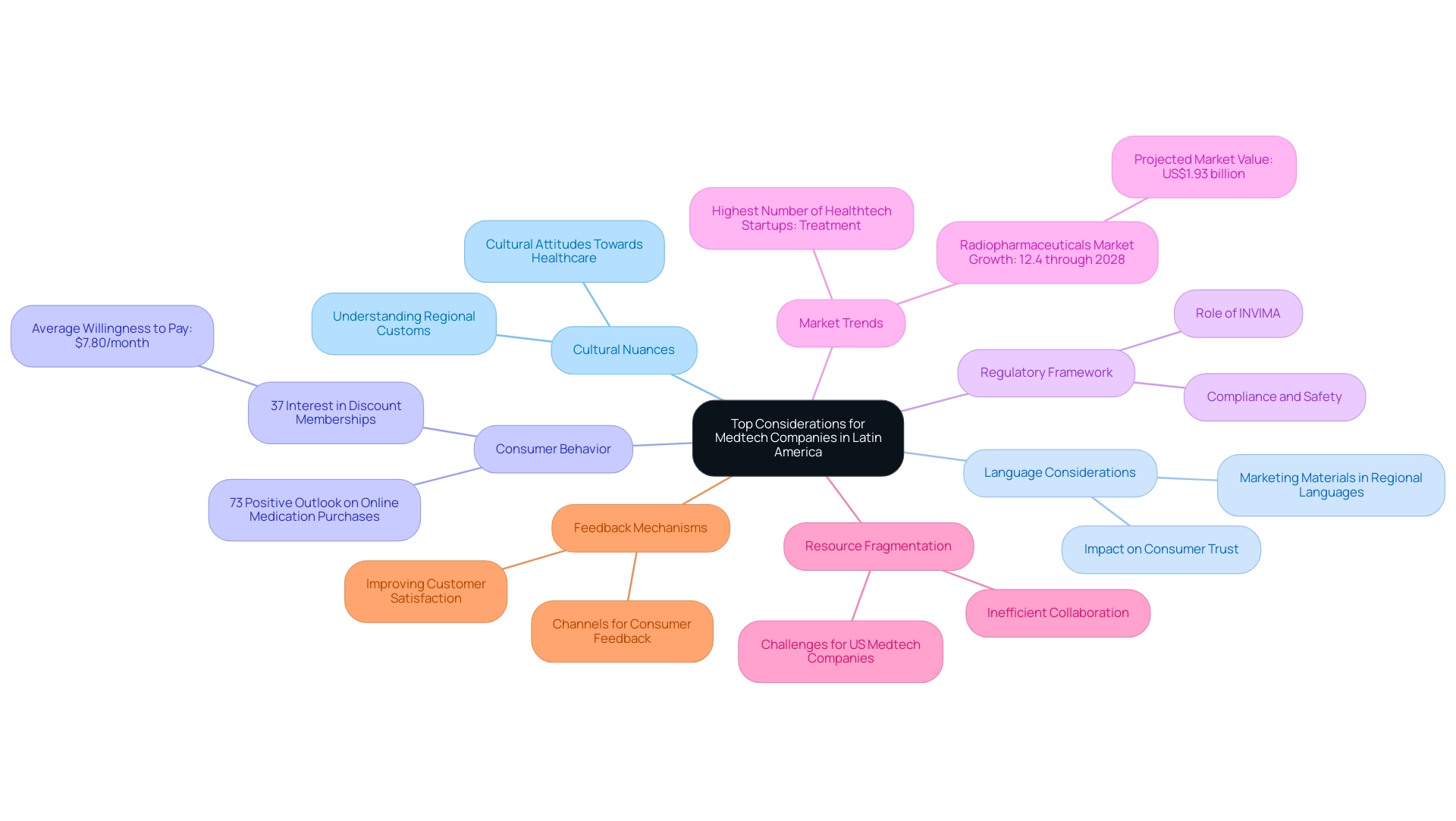
Language Considerations: Ensuring that marketing materials and product information are available in regional languages enhances both accessibility and comprehension.
This is crucial, as studies indicate that language accessibility significantly influences consumer trust and engagement in healthcare marketing. Moreover, addressing language barriers is one of the top considerations for Medtech companies entering Latin America, as they can hinder effective communication between US Medtech companies and regional stakeholders, complicating the execution of clinical trials.
Cultural Sensitivity: A deep understanding of cultural attitudes towards healthcare and medical devices can shape effective marketing strategies and product designs.
Understanding the top considerations for medtech companies entering Latin America, including regional customs and values, is key to resonating with consumers in diverse markets.
Consumer Behavior: Analyzing local consumer behavior—such as purchasing habits and preferences—provides insights for effective product positioning. A recent survey revealed that 73% of respondents had a positive outlook on online medication purchases, underscoring the trend toward digital channels in healthcare.
Additionally, 37% of respondents expressed interest in a medication and lab test discount membership, which reflects some of the top considerations for medtech companies entering Latin America to cater to these preferences.
Regulatory Framework: Understanding the role of INVIMA as a Level 4 health authority is vital, as it ensures the compliance and safety of medical devices in the market. Establishing trust and credibility with consumers and healthcare providers alike is one of the top considerations for medtech companies entering Latin America, making regulatory oversight essential.
Market Trends: The segment with the highest number of Healthtech startups in South America in 2023 is treatment, highlighting a growing area of opportunity for innovation and investment in the medtech sector.
- Resource Fragmentation: The fragmentation of resources and the absence of CRO corporate structures in South America pose significant challenges for US Medtech companies. These issues can lead to inefficient collaboration and hinder the successful implementation of clinical research.
Feedback Mechanisms: Establishing robust channels for consumer feedback allows companies to adapt their offerings to better align with local needs. This responsiveness not only improves customer satisfaction but also cultivates long-term loyalty in a competitive environment.
As the medtech sector continues to progress in South America, the Top Considerations for Medtech Companies Entering Latin America will include addressing cultural nuances and consumer preferences, along with a strong understanding of compliance dynamics and existing challenges, which will be crucial for success.
Identifying and Mitigating Market Entry Risks
Reducing market entry risks in South America requires a multifaceted strategy, and one of the top considerations for medtech companies entering Latin America is grasping the compliance environment. For instance, INVIMA (Colombia National Food and Drug Surveillance Institute) plays a pivotal role in overseeing the marketing and manufacturing of health products. Established in 1992, INVIMA is responsible for ensuring compliance with health standards and implementing best practices for the import and export of medical devices.
Its classification as a Level 4 health authority by PAHO/WHO underscores its competence in guaranteeing the safety, efficacy, and quality of health products.
Here are essential strategies:
-
Economic Volatility: Understanding the economic landscape is critical, especially as inflation persists in various Latin American countries, raising concerns about price stability. The projected increase of the population aged 65 and older to 20.6% by 2053 underscores the urgency for stable healthcare solutions.
Companies must analyze these economic conditions and potential fluctuations to better prepare for financial uncertainties.
-
Regulatory Changes: The medtech sector must stay alert concerning compliance updates. Recent reforms, such as those championed by President Lacalle Pou in Uruguay, which raised the retirement age to 65 and allowed retirees to continue working, illustrate the shifting regulatory environment aimed at modernizing systems.
Staying informed about these changes can prevent compliance issues and operational disruptions, ensuring smoother entry into the industry.
-
Compliance Best Practices: Engaging with INVIMA requires adherence to specific best practices, such as thorough documentation of product specifications and clinical data, timely submission of applications, and proactive communication with INVIMA officials.
Companies should also familiarize themselves with INVIMA's guidelines and participate in training sessions or workshops to enhance compliance understanding.
-
Competitive Analysis: A thorough competitive analysis is essential to identify key industry players and potential challenges. Companies can utilize insights from case studies, particularly on wealth taxes as tools for economic reform, to understand the competitive landscape and anticipate shifts in industry dynamics.
These wealth taxes have gained prominence as a means to reduce inequality and generate resources for combating global warming, which can significantly influence company strategies.
-
Political Stability: Assessing the political landscape is essential for understanding governance and policy risks. With borrowing costs for issuers in South America and the Caribbean widening significantly—evidenced by LAC bond spreads reaching 471 basis points compared to the overall EMBIG at 305 basis points—companies must analyze political stability to navigate potential governance challenges effectively.
These statistics provide a concrete example of the financial landscape and its implications for market entry, emphasizing the top considerations for medtech companies entering Latin America.
By integrating these strategies, firms can better position themselves to enter the Latin American market with a nuanced understanding of the associated risks and opportunities, particularly considering INVIMA's critical role in medtech regulation and its impact on local economies through job creation and healthcare improvement.
Additionally, understanding the specific functions of the Directorate for Medical Devices and other Technologies within INVIMA can further enhance compliance and operational strategies.
Conclusion
Entering the Latin American medtech market requires a well-rounded strategy that addresses key factors such as regulatory compliance, thorough market research, and the establishment of strong distribution channels. Companies must prioritize understanding the diverse regulatory landscapes across different countries, as compliance is not merely a formality but a critical component that can determine market success or failure.
Moreover, engaging in comprehensive market research is essential for identifying local healthcare needs and consumer preferences. This knowledge is pivotal for crafting effective pricing strategies and positioning products in a manner that resonates with both healthcare providers and consumers. Establishing robust distribution partnerships further enhances market access and facilitates smoother entry into the dynamic Latin American landscape.
Cultural nuances and local consumer behaviors play significant roles in shaping successful marketing strategies. Companies that prioritize language accessibility, cultural sensitivity, and feedback mechanisms are more likely to build trust and foster long-term relationships with their target audiences. Additionally, forming strategic partnerships with local distributors, healthcare providers, and research institutions can provide invaluable insights and facilitate compliance with local regulations.
As the medtech sector in Latin America continues to grow, organizations must remain vigilant in identifying and mitigating market entry risks. By understanding economic volatility, regulatory changes, and the competitive landscape, firms can navigate potential challenges and seize opportunities in this burgeoning market. Ultimately, a holistic approach that combines compliance, market understanding, and strategic collaboration is essential for achieving sustainable success in the Latin American medtech industry.
Frequently Asked Questions
What are the key factors for successful entry into the South American market for medtech companies?
Successful entry necessitates a strategic approach focusing on regulatory compliance, comprehensive industry analysis, and regional knowledge.
Why is regulatory compliance important for medtech companies in South America?
Navigating regional regulations is crucial as each Latin American nation has unique requirements for medical devices. Non-compliance can lead to significant obstacles, including delays or exclusion from the market.
What should medtech companies consider regarding industry analysis?
Companies should conduct a comprehensive analysis to understand healthcare requirements, competitive dynamics, and pricing strategies, which facilitate effective product positioning and acceptance among consumers and healthcare providers.
How do distribution channels impact market entry?
Establishing the right distribution partnerships is vital for accessing healthcare providers and end-users, significantly influencing the reach and success of medical devices in the market.
What is the role of regional knowledge in entering the South American market?
Working with regional specialists and advisors provides invaluable insights into industry dynamics and regulatory requirements, enhancing a company's strategy and compliance efforts.
What are the implications of anti-corruption policies for medtech companies?
Prioritizing strong anti-corruption policies is essential due to ongoing challenges in the region, with recent legislation emphasizing the necessity for robust compliance measures.
What is involved in the process of advancing medical device trials in Colombia?
The process includes feasibility studies, site selection, principal investigator selection, trial setup, and obtaining approvals from INVIMA and ethics committees, as well as project management and monitoring.
How can companies ensure compliance during clinical trials in South America?
Companies should conduct regional clinical trials with a thorough understanding of local ethical standards and protocols, and utilize services that cover compliance reviews and documentation alignment.
What changes are being made to the approval processes for medical devices?
Regulatory agencies are transitioning to online submissions for medical device registrations, enhancing the efficiency of the registration process, though the pace varies by country.
How does participation in clinical trials affect local economies?
Conducting clinical trials supports local economies by creating jobs and advancing healthcare, while agreements like MERCOSUR facilitate trade and collaboration in the region.




