Overview
The article addresses the critical steps and considerations necessary for designing effective medical device trials in Colombia. It underscores the significance of comprehending local regulatory frameworks and actively engaging with stakeholders. Key components such as:
- Device classification
- Regulatory submissions
- Ethics approval
- Best practices for trial management
are outlined. Adhering to these guidelines not only enhances compliance but also significantly improves the likelihood of successful outcomes within the Colombian healthcare landscape.
Introduction
In the rapidly evolving landscape of medical device trials, understanding the regulatory environment is crucial for success, particularly in Colombia. With the National Food and Drug Surveillance Institute (INVIMA) at the helm, researchers must navigate a complex framework that dictates everything from device classification to compliance with international standards.
As Colombia's healthcare market expands—driven by a growing aging population and increasing demand for innovative medical technologies—the stakes are higher than ever.
This article delves into the essential elements of conducting medical device trials in Colombia, exploring the regulatory requirements, best practices, and collaborative strategies that can lead to successful outcomes in this dynamic field.
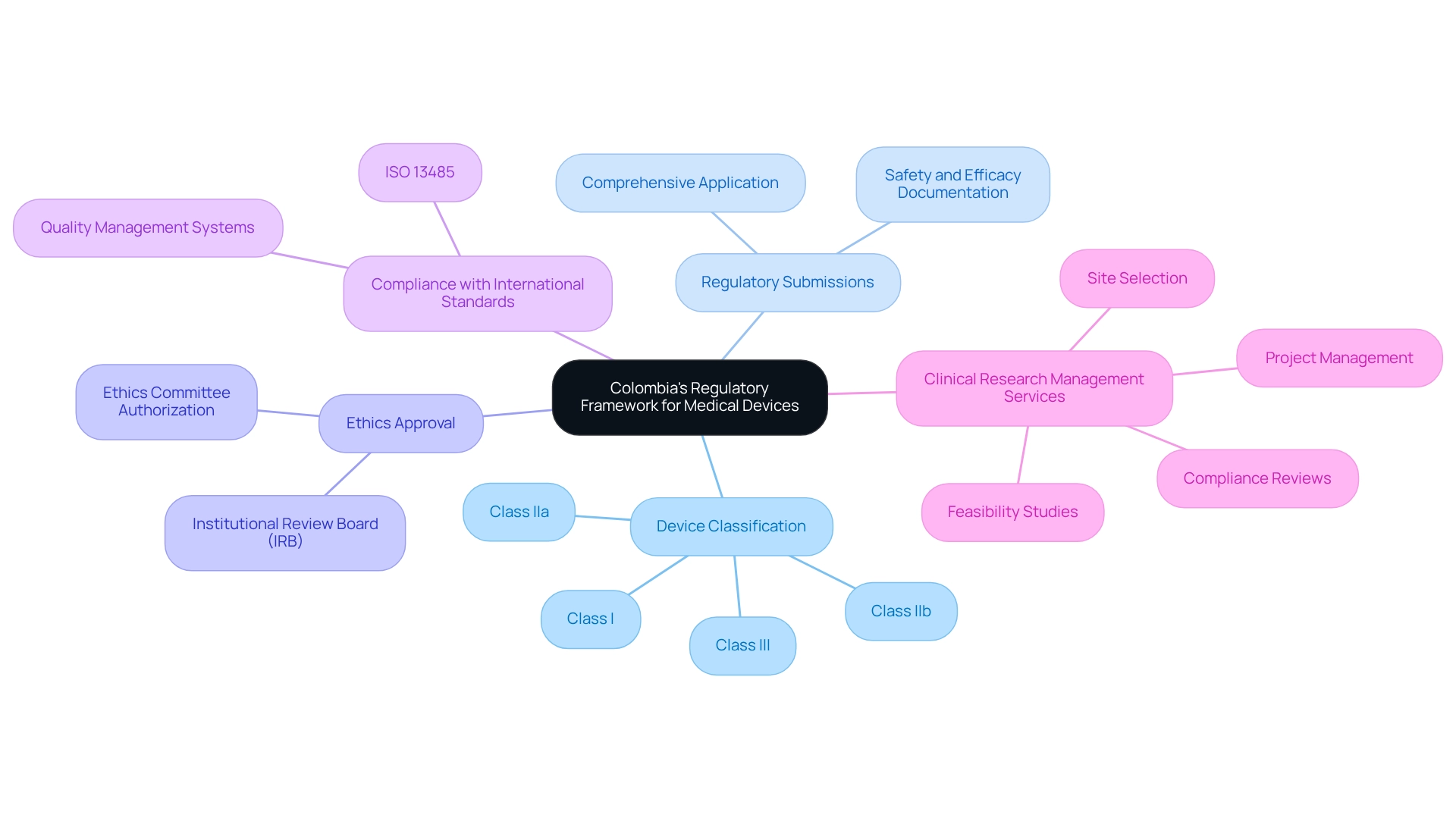
Understanding Colombia's Regulatory Framework for Medical Devices
A comprehensive grasp of the regulatory structure set by the National Food and Drug Surveillance Institute (INVIMA) is essential for effective medical device trial design in Colombia. This framework is crucial for ensuring compliance and facilitating successful outcomes. Key components include:
-
Device Classification: Medical devices in Colombia are categorized into four classes (Class I, IIa, IIb, and III) based on their associated risks. Each classification involves unique regulatory requirements and approval timelines, which are essential for planning research protocols effectively.
-
Regulatory Submissions: Before starting an experiment, researchers must submit a comprehensive application to INVIMA. This application must include detailed documentation regarding the device's safety, efficacy, and manufacturing processes, ensuring that all necessary information is available for regulatory review.
-
Ethics Approval: All medical studies are required to obtain authorization from an Institutional Review Board (IRB) or an ethics committee. This step is essential to safeguard the rights and welfare of participants, aligning with ethical standards in clinical research.
-
Compliance with International Standards: Adhering to international guidelines, such as ISO 13485 for quality management systems, is often mandated. This compliance demonstrates a commitment to safety and efficacy, which is critical for gaining regulatory approval.
-
Comprehensive Clinical Research Management Services: Engaging with a service provider like bioaccess can streamline the research process. Their capabilities encompass feasibility studies, site selection, compliance reviews, experimental setup, import permits, project management, and reporting on study status and adverse events. This comprehensive method guarantees that all facets of the trial are handled effectively and in line with INVIMA regulations.
Grasping these regulatory components is essential for navigating the intricacies of medical device trial design in Colombia. The country's steady economic growth, coupled with a growing aging population and increasing demand for healthcare services, underscores the importance of efficient regulatory processes. For instance, the market for diagnostic imaging equipment is projected to expand significantly, driven by advancements in technology and a rise in routine diagnostic screenings.
In 2021, Colombia noted an average of 6.0 CT scans per million inhabitants, emphasizing the growing dependence on such technological advancements. As stated by Fortune Business Insights, elements like technological progress and increasing product authorizations worldwide are anticipated to propel the acceptance of these items. By understanding INVIMA's regulations and the wider healthcare environment, you can strategically plan medical device trial design in Colombia that adheres to local standards while addressing the changing demands of the healthcare market.
Moreover, the existing environment offers chances for innovation, collaboration, and growth in healthcare services, creating an exciting period for research in healthcare technology in Colombia.
Key Requirements for Conducting Medical Device Trials in Colombia
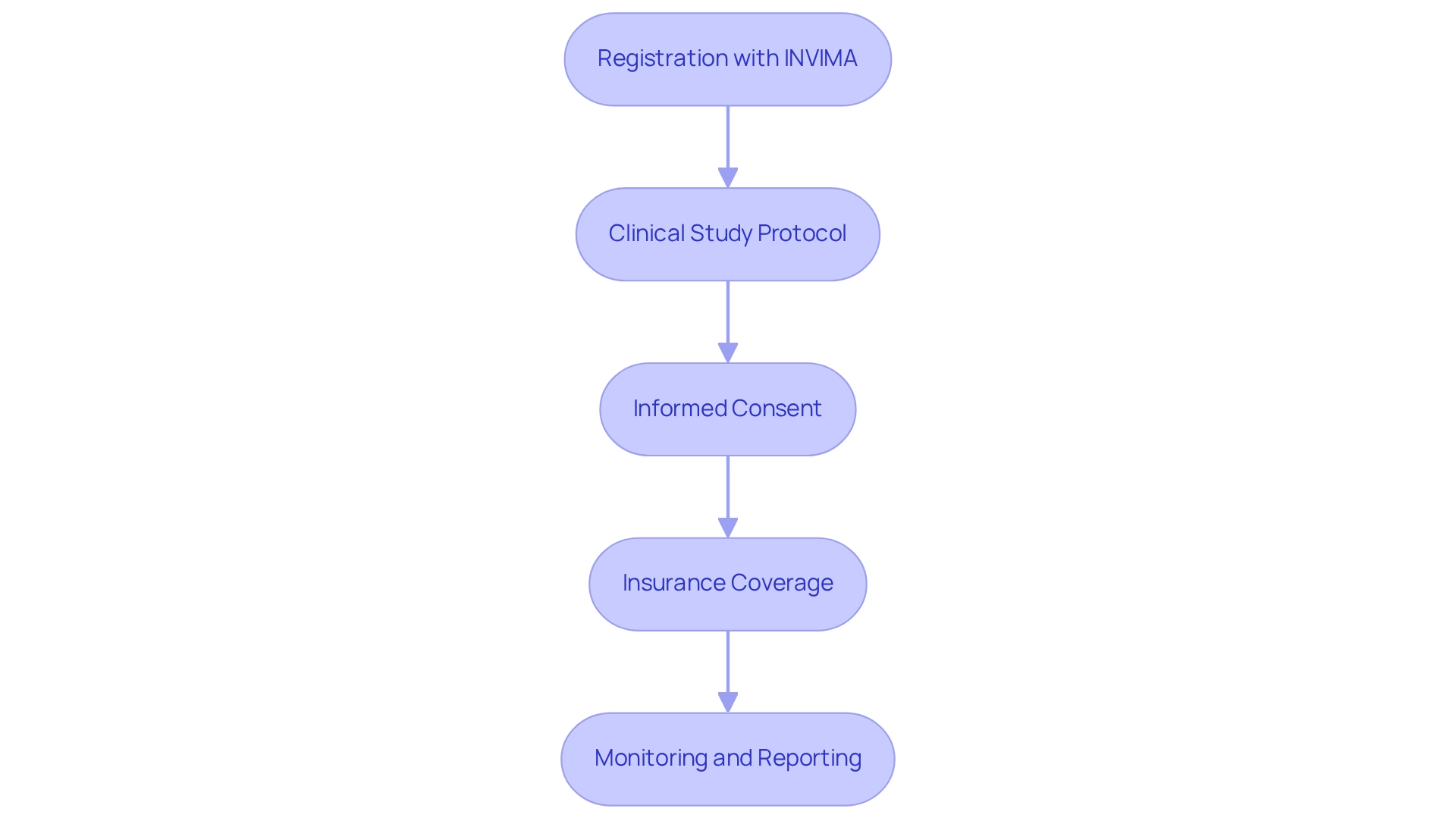
Carrying out healthcare equipment evaluations in Colombia necessitates adherence to several essential stipulations that ensure conformity and facilitate a successful study:
-
Registration with INVIMA: Prior to initiating any research study, it is imperative that all medical instruments are registered with INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos). This process mandates the submission of a Certificate of Free Sale (CFS) or a Certificate to Foreign Government (CFG), which verifies the device's approval for sale in its country of origin. Notably, Class III IVDs have a 5-year renewal period under INVIMA registration guidelines, a crucial consideration for planning your study's timeline.
-
Clinical Study Protocol: A thorough clinical study protocol is vital. This document should delineate the study design, objectives, methodology, and statistical analysis plan, ensuring meticulous planning and documentation of all experimental elements.
-
Informed Consent: Securing informed consent from all study participants is essential. This process involves clearly communicating the risks and benefits associated with participation, ensuring that participants are fully informed about the study's scope.
-
Insurance Coverage: Adequate insurance must be procured to address potential liabilities that may arise during the proceedings, protecting both participants and researchers from unforeseen circumstances.
-
Monitoring and Reporting: Continuous observation of the study is required, with regular updates to INVIMA. This includes documenting any adverse events or deviations from the established protocol, which is crucial for maintaining the study's integrity.
By diligently adhering to these requirements, researchers can navigate the complexities of medical device trial design in Colombia more effectively, ensuring compliance with local regulations and enhancing the likelihood of favorable outcomes. Furthermore, as highlighted by the collaboration between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, there is a concerted effort to position Barranquilla as a leading hub for clinical research in Latin America. This initiative is particularly timely, considering that Colombia's aging population is projected to represent 27.5% of the total demographic by 2050, amplifying the demand for innovative healthcare solutions.
Additionally, the recent roadmap released by the US FDA for regulatory guidance topics in 2023 underscores the evolving regulatory landscape, which is pertinent for conducting studies in Colombia. For practical insights, addressing frequently asked questions regarding the registration process, as detailed in the case study titled 'Common Questions Regarding Colombia Device Registration,' can further assist manufacturers in adequately preparing and complying with local regulations.
Essential Elements of Medical Device Trial Design
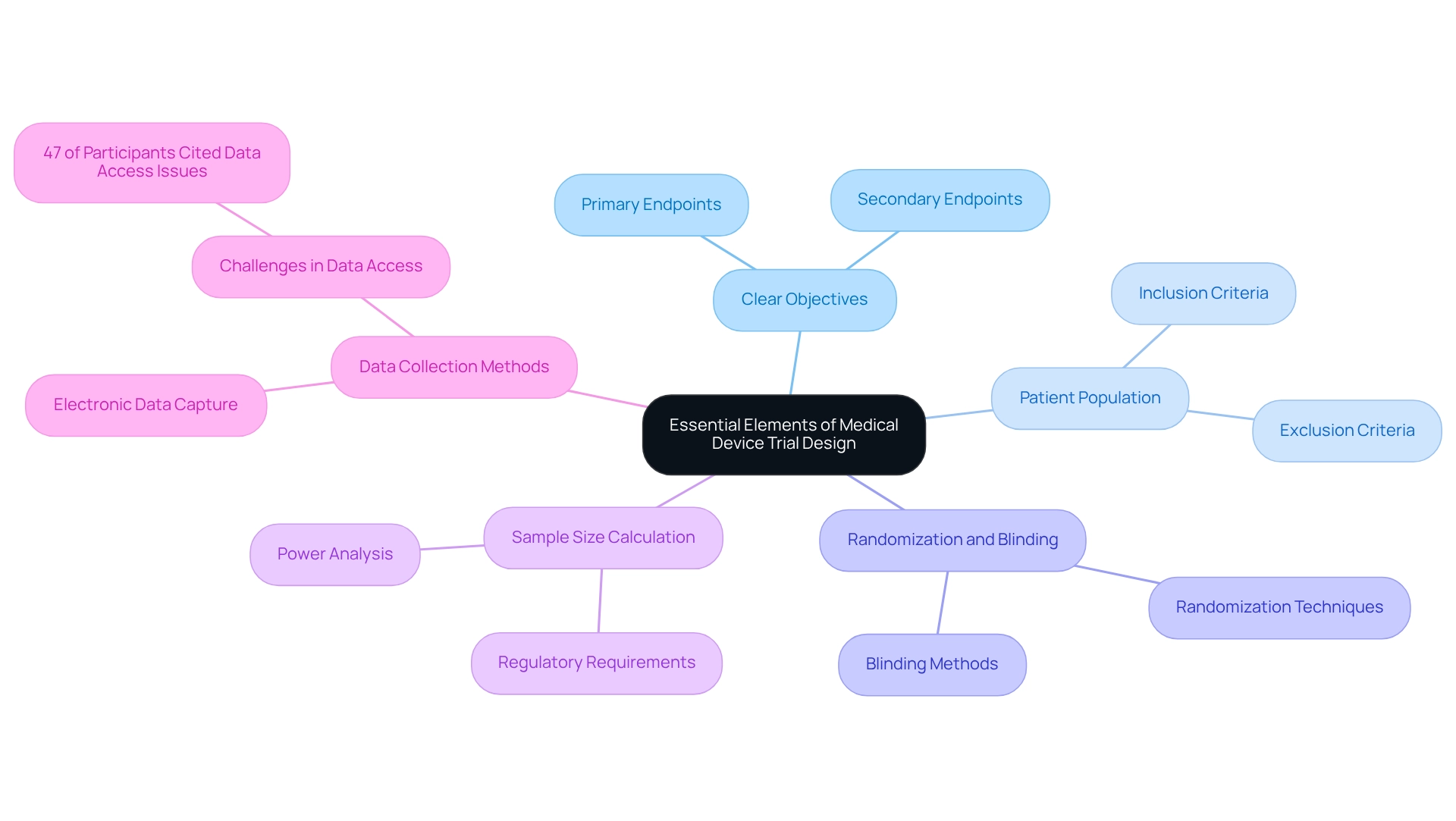
Creating effective medical equipment studies necessitates a meticulous approach that incorporates several critical components:
- Clear Objectives: Establishing specific, measurable primary and secondary endpoints is vital. These objectives must closely align with the intended use of the device, ensuring that the focus of the test remains relevant and actionable. As noted by PG, an Editorial Board member, clear objectives are fundamental to the success of clinical trials.
- Patient Population: A clearly defined target patient group is essential for the validity of study results. Inclusion and exclusion criteria must be meticulously crafted to reflect the demographics of the intended user base, particularly in regions like Colombia, where the design of medical device trials can significantly influence outcomes.
- Randomization and Blinding: To mitigate bias, implementing randomization is crucial. Furthermore, considering blinding techniques can further enhance the integrity of the study results, ensuring that the data collected is as objective as possible.
- Sample Size Calculation: Conducting a thorough sample size calculation is necessary to ensure that the study is adequately powered. This step is essential for identifying significant differences in results, especially given the regulatory requirements for clinical evidence across all categories of healthcare products, including Class I, as mandated by the EU.
- Data Collection Methods: Establishing robust data collection methods is imperative for ensuring accuracy and reliability. Utilizing electronic data capture systems can streamline this process, facilitating better data management and analysis. Nonetheless, challenges exist; a recent study discovered that 47% of participants identified data access as the primary obstacle during the OPC generation process.
By focusing on these fundamental aspects, researchers can create studies that not only yield high-quality data but also facilitate successful regulatory submissions. Involving stakeholders throughout the process, as emphasized in the case study on stakeholder engagement in OPC development, can further enhance the relevance and applicability of the results, ultimately benefiting the assessment and creation of medical devices. At bioaccess®, we emphasize extensive research study management services, drawing on over 20 years of expertise in Medtech.
Our offerings encompass Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies. Our proficiency in managing the intricacies of medical studies in Latin America ensures that your research is conducted efficiently and effectively. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, attests to our capabilities by sharing his positive experience with bioaccess® during his initial human study in Colombia, underscoring our commitment to excellence in medical device trial design in Colombia.
Moreover, we possess in-depth knowledge of the regulatory landscape in Colombia, particularly concerning medical device trial design, ensuring adherence to INVIMA standards and facilitating smoother testing processes.
Navigating Challenges in Medical Device Trials
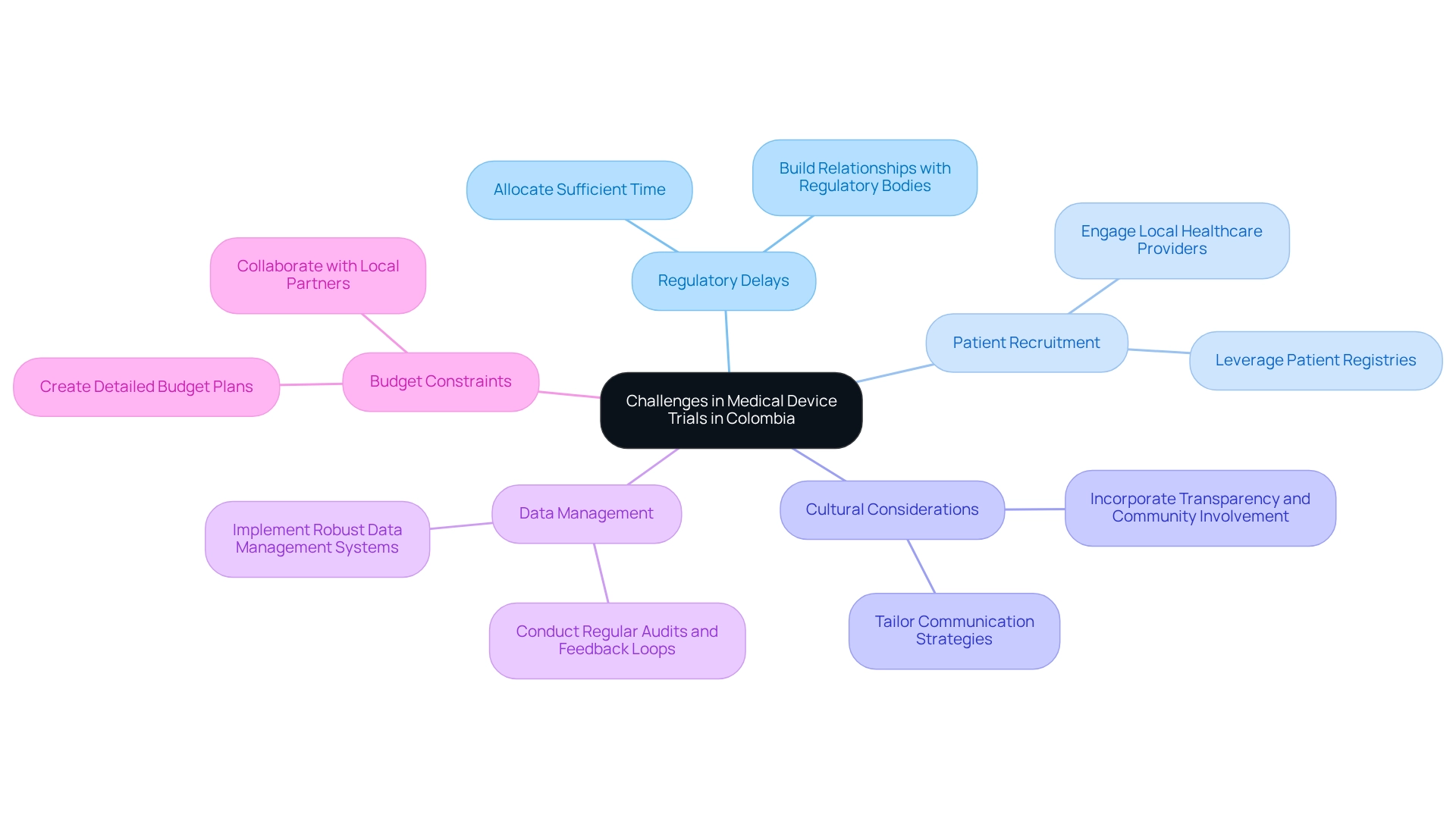
Carrying out equipment trials in Colombia presents several challenges that require strategic planning and local insights.
-
Regulatory Delays: The regulatory landscape in Colombia can lead to significant delays in obtaining necessary approvals. To mitigate this, researchers should anticipate potential setbacks by allocating sufficient time for submissions and responses. Colombia's health authority, INVIMA, designated as a Level 4 authority by PAHO/WHO, plays an essential role in overseeing and classifying health instruments. Understanding local regulatory processes is essential to streamline approvals, and establishing strong relationships with regulatory bodies can facilitate smoother interactions and quicker resolutions.
-
Patient Recruitment: Recruiting suitable participants for specialized medical devices can be particularly challenging. Engaging local healthcare providers and leveraging patient registries can enhance recruitment efforts. Notably, Colombia's diverse population offers a larger subject pool, which can facilitate faster recruitment rates compared to other regions. The hiring crisis in the U.S. has led numerous manufacturers to contemplate performing tests abroad, where they can take advantage of simpler recruitment and lower expenses. This context emphasizes the benefits of medical device trial design in Colombia.
-
Cultural Considerations: A profound comprehension of local cultural perspectives regarding medical devices and research studies is essential for effective participant involvement and retention. Tailoring communication strategies to resonate with local communities can foster trust and encourage participation. Incorporating foundational elements in medical research, such as transparency and community involvement, can further enhance trust among stakeholders.
-
Data Management: Ensuring precise and uniform data collection is essential for the integrity of clinical studies. Implementing robust data management systems and providing comprehensive training for staff can help address potential data-related challenges, ensuring compliance and reliability in results. Regular audits and feedback loops can also improve data quality and adherence to protocols. bioaccess® is committed to ensuring information security and client trust, addressing concerns through established grievance and data protection procedures.
-
Budget Constraints: Clinical studies can incur significant costs, and unexpected expenses may arise. Creating a detailed budget that considers all possible expenses and obtaining sufficient financing in advance is crucial for the successful implementation of tests. Working together with local partners can also assist in recognizing affordable solutions and resources.
By actively tackling these issues and incorporating strategies like those mentioned, researchers can improve the chances of positive results in the medical device trial design in Colombia. As Julio G. Martinez-Clark, CEO of bioaccess Colombia, observes, the medical device trial design in Colombia benefits from a sizable and varied population, knowledgeable clinical research sites, and effective regulatory processes, making it an appealing option for U.S. healthcare companies seeking to carry out clinical research.
The Role of Post-Market Surveillance in Medical Device Trials
Post-market monitoring is essential for ensuring the safety and efficacy of medical instruments throughout their lifecycle. This process encompasses several key components:
-
Monitoring Equipment Performance: Once a product is available, continuous monitoring is vital to assess its performance in real-world settings. This helps identify any adverse events that may arise, ensuring that any issues are promptly addressed.
-
Data Gathering: Establishing robust systems for gathering information on equipment usage and patient outcomes is critical for effective post-market monitoring. This information not only assists in evaluating equipment performance but also facilitates regulatory compliance and guides future research.
-
Regulatory Reporting: Manufacturers must adhere to stringent regulatory standards for reporting adverse occurrences and product failures to INVIMA, Colombia's National Institute for Drug and Food Surveillance. As a Level 4 health authority recognized by PAHO/WHO, INVIMA plays a crucial role in maintaining market authorization and ensuring patient safety through its oversight of health products. Compliance with these regulations is essential for manufacturers operating in the Colombian market.
-
Continuous Improvement: Insights gained from post-market monitoring can drive changes and upgrades to health equipment. By analyzing performance data and user feedback, manufacturers can make informed decisions that enhance product safety and efficacy.
-
Stakeholder Engagement: Actively engaging with healthcare providers and patients is crucial for gathering valuable feedback on equipment performance and user experience. With 3,464 hospitals in Colombia as of 2022, the landscape presents significant opportunities for feedback from a broader healthcare network. This collaboration can lead to enhancements in equipment design and functionality, ultimately benefiting patient outcomes. In 2025, the significance of post-market monitoring is underscored by the competitive nature of the Colombian healthcare equipment market, which includes major players like Medtronic, Abbott, Stryker, and Johnson & Johnson Services, Inc. A structured approach to post-market monitoring, relevant to medical device trial design in Colombia, ensures that such products meet rigorous standards of safety, efficacy, and quality.
This not only creates a smoother pathway for manufacturers but also boosts overall confidence in healthcare products among professionals and patients alike. As Alvaro Enrique Rincon Mautner from SPI Americas emphasizes, robust post-market surveillance practices are crucial in navigating the challenges posed by this mature and competitive market. At bioaccess, we are committed to supporting research studies that comply with these stringent post-market monitoring practices, ensuring that our clients can navigate the complexities of the Colombian healthcare landscape efficiently.
Best Practices for Managing Medical Device Clinical Trials
To effectively manage medical device clinical trials, particularly in Latin America, it is essential to adopt the following best practices:
- Establish Clear Communication: Open lines of communication among all stakeholders—including investigators, sponsors, and regulatory bodies—are crucial for ensuring alignment and transparency throughout the research process. Effective communication not only fosters collaboration but also mitigates misunderstandings that can lead to delays or complications. Significantly, a recent survey showed that 49% of participants indicated a wish to comprehend the length of studies, highlighting the necessity of clear and accessible communication about study details. Additionally, the sentiment conveyed by Participant UR13, "We’re on your side," emphasizes the importance of effective communication and support from study personnel.
- Implement a Robust Project Management Plan: A comprehensive project management plan is vital for outlining timelines, responsibilities, and milestones. This organized method helps maintain the process on course and permits proactive modifications as necessary. Studies show that clearly outlined project management strategies greatly improve success rates in clinical studies, especially regarding medical device trial design in Colombia. At bioaccess®, we specialize in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring that each study is meticulously planned and executed.
- Training and Support: Comprehensive training for all experimental staff is imperative. Ensuring that team members comprehend their roles, responsibilities, and the regulatory environment can greatly improve efficiency. This training should also encompass conflict management and emotional intelligence, as these skills are increasingly recognized as essential for a competent translational research workforce. Additionally, incorporating competency-based communication training for clinical research professionals can further improve team dynamics and participant interactions.
- Regular Monitoring and Auditing: Conducting regular monitoring and auditing of experimental processes is essential for identifying and addressing issues promptly. This proactive strategy not only aids in upholding adherence to regulatory standards but also improves the overall quality of the study data. bioaccess® employs rigorous monitoring practices to ensure that all studies meet the highest standards of compliance and quality.
- Utilize Technology: Leveraging technology for data collection, monitoring, and reporting can significantly enhance both efficiency and accuracy. The incorporation of advanced data management systems enables real-time insights and streamlined communication, which are essential for successful project management. At bioaccess®, we employ advanced technology to enable efficient data management and reporting throughout the research process.
By following these best practices, researchers can enhance the oversight of their medical studies, particularly in the context of medical device trial design in Colombia, thereby boosting the chances of successful results. Moreover, case studies have demonstrated that experiments with effective communication strategies result in greater participant engagement and satisfaction, ultimately aiding the success of the study. The results from the case study named 'Interest in Clinical Trials' highlight the significance of available research study data, especially concerning participant preferences for details about study purpose, length, and safety outcomes.
Furthermore, with more than 20 years of experience in the Medtech sector, bioaccess® showcases the adaptability and knowledge required to manage the intricacies of clinical studies effectively.
Collaborating with Local Stakeholders for Trial Success
Working together with local stakeholders is vital for the success of medical device trial design in Colombia. Here are key strategies to enhance trial effectiveness:
-
Engage Local Investigators: Collaborating with local investigators who possess a thorough knowledge of the healthcare environment greatly enhances study design and execution. Their familiarity with regional practices and patient populations allows for more tailored and effective study protocols. As bioaccess® aims to advance medical device trial design in Colombia sooner through its expertise and customized approach, engaging local investigators is a vital step in this process.
-
Leverage Local Networks: Utilizing local networks is essential for facilitating patient recruitment and retention. These networks assist in guaranteeing a varied participant pool, which is crucial for the generalizability of study results. Successful patient recruitment via local connections has been demonstrated to improve enrollment rates, with one researcher estimating that a study would need approximately 140 patients to obtain significant results. This statistic underscores the importance of strategic recruitment efforts.
-
Build Relationships with Regulatory Bodies: Establishing strong relationships with INVIMA and other regulatory authorities can streamline the approval process and ensure compliance with local regulations. This proactive involvement reduces potential delays and promotes a smoother route to case initiation. The backing from Colombia's Minister of Health further highlights the significance of regulatory cooperation in improving the clinical research environment.
-
Engage Community Organizations: Partnering with community organizations can enhance awareness about the initiative and its advantages, nurturing community backing. These organizations serve as valuable allies in disseminating information and encouraging participation among local populations.
-
Feedback Mechanisms: Implementing feedback mechanisms to gather insights from local stakeholders is essential. This method enables researchers to make informed modifications to study protocols based on real-world experiences, ultimately enhancing outcomes and participant satisfaction. As Stephanie Pugh, PhD at NRG Oncology Statistics and Data Management Center, stated, "Communication is key" in ensuring effective collaboration.
-
Routine Pilot Studies: Carrying out routine pilot studies prior to comprehensive studies enhances study designs and tackles methodological weaknesses in healthcare research. This practice aligns with the aim of improving study effectiveness and can result in more robust outcomes, as demonstrated by bioaccess®'s commitment to managing various study types, including Early-Feasibility Studies and First-In-Human studies.
-
Surrogate Endpoints: Employing surrogate endpoints in medical studies can accelerate the process by delivering earlier outcomes associated with treatment effectiveness. This method can shorten the duration and expenses, but it is essential that these endpoints are verified to confirm they align with real-world health results.
-
Economic Impact: The execution of experimental studies significantly affects local economies, including job creation and healthcare enhancement. By fostering collaboration with local stakeholders, researchers can enhance the effectiveness and efficiency of their clinical trials, paving the way for successful medical device trial design in Colombia and contributing to local economic growth.
Conclusion
The regulatory landscape for medical device trials in Colombia presents both challenges and opportunities that require careful navigation. Understanding the framework established by INVIMA is paramount, as it encompasses essential elements such as device classification, regulatory submissions, and compliance with international standards. By adhering to these guidelines, researchers can streamline their processes and enhance the likelihood of successful outcomes in this expanding market.
Moreover, addressing key requirements, such as obtaining informed consent and ensuring proper insurance coverage, is critical for maintaining ethical standards and participant safety. The incorporation of robust project management practices and effective communication strategies can further bolster the efficiency of clinical trials. Engaging local stakeholders and leveraging their insights not only enhances trial design but also fosters community support, ultimately improving participant recruitment and retention.
As the demand for innovative medical technologies continues to rise, particularly with Colombia's aging population, the importance of conducting well-structured medical device trials cannot be overstated. By embracing best practices and collaborating with local entities, researchers can contribute to the advancement of healthcare solutions that meet the needs of the population.
In conclusion, the landscape for medical device trials in Colombia is ripe with potential. By navigating the regulatory complexities with diligence and fostering collaborative relationships, researchers can pave the way for successful innovations that enhance patient care and support the growth of the healthcare sector in the region.
Frequently Asked Questions
What is the importance of understanding INVIMA's regulatory structure for medical device trials in Colombia?
A comprehensive understanding of INVIMA's regulatory structure is essential for effective medical device trial design in Colombia, ensuring compliance and facilitating successful outcomes.
How are medical devices classified in Colombia?
Medical devices in Colombia are classified into four classes (Class I, IIa, IIb, and III) based on their associated risks, with each classification having unique regulatory requirements and approval timelines.
What must researchers submit to INVIMA before starting a medical device trial?
Researchers must submit a comprehensive application to INVIMA that includes detailed documentation regarding the device's safety, efficacy, and manufacturing processes.
Is ethics approval required for medical studies in Colombia?
Yes, all medical studies in Colombia must obtain authorization from an Institutional Review Board (IRB) or an ethics committee to safeguard the rights and welfare of participants.
What international standards must be complied with in medical device trials?
Compliance with international guidelines, such as ISO 13485 for quality management systems, is often mandated to demonstrate a commitment to safety and efficacy.
How can engaging with a service provider like bioaccess benefit medical device trials?
Engaging with a service provider like bioaccess can streamline the research process by offering capabilities such as feasibility studies, site selection, compliance reviews, experimental setup, import permits, project management, and reporting on study status and adverse events.
What is the significance of Colombia's economic growth and aging population for medical device trials?
Colombia's steady economic growth and increasing demand for healthcare services, driven by a growing aging population, underscore the importance of efficient regulatory processes for medical device trials.
What are the key requirements for conducting healthcare equipment evaluations in Colombia?
Key requirements include registration with INVIMA, a thorough clinical study protocol, securing informed consent from participants, obtaining adequate insurance coverage, and continuous monitoring and reporting of the study.
What is the role of the Certificate of Free Sale (CFS) in the registration process with INVIMA?
The CFS verifies the device's approval for sale in its country of origin and is required for the registration of all medical instruments with INVIMA.
How does the recent roadmap released by the US FDA relate to conducting studies in Colombia?
The roadmap underscores the evolving regulatory landscape, which is pertinent for conducting studies in Colombia and may provide practical insights for researchers.

