Introduction
The medtech clinical trial landscape in the Dominican Republic is rapidly evolving, positioning the country as an attractive destination for international sponsors. With a favorable regulatory framework and a diverse pool of qualified research participants, the Dominican Republic is set to conduct approximately 15 clinical trials in 2024, reflecting its growing significance in the global medtech sector. Recent governmental reforms have streamlined approval processes, enabling Contract Research Organizations (CROs) to execute trials more efficiently.
This article delves into the essential dynamics of conducting medtech studies in the region, highlighting the importance of regulatory compliance, community engagement, and strategic partnerships. By understanding these critical factors, CROs can navigate the complexities of the landscape and leverage the numerous advantages available, ultimately contributing to enhanced healthcare outcomes and economic growth.
Understanding the Medtech Clinical Trial Landscape in the Dominican Republic
The medtech research landscape in the Dominican Republic is becoming increasingly attractive to international sponsors, due to a favorable regulatory environment and a strong pool of qualified research participants. In 2024, the Dominican Republic is projected to conduct approximately 15 clinical studies, underscoring its emerging significance in the medtech sector. This growth is supported by recent governmental reforms aimed at streamlining approval processes, which facilitate efficient conduct for Contract Research Organizations (CROs). Furthermore, the presence of well-established healthcare facilities and a diverse patient demographic greatly enhances the feasibility of conducting medtech studies. It is essential for CROs to comprehend these dynamics to navigate the landscape effectively and achieve successful outcomes.
Along with operational benefits, remaining informed about the changing regulations and guidelines overseeing medical studies in the Dominican Republic is essential. Familiarity with these regulations ensures compliance and maintains research integrity. Potential clients should prioritize CROs that demonstrate a profound understanding of local regulations and possess the expertise necessary to manage the complexities involved in executing medtech studies in this region. As highlighted by a participant from URC7, "I think that here, as a country…the problem is not finding who will fund you. The problem is finding who can write the proposal." This underscores the importance of skilled proposal writing in securing funding for clinical trials.
Moreover, the case analysis titled 'Community Engagement in Research' illustrates the importance of involving local communities in the research process. While resistance to community involvement has been noted, with concerns about communities' capability to contribute to complex research designs, acknowledging the significance of sharing results with the community is essential. This highlights the need for appropriate methodologies to enhance community involvement and ensure transparency in research outcomes, ultimately contributing to job creation, economic growth, and improved healthcare in the region. Additionally, the impact of medtech research on local economies creates ripples with far-reaching benefits, including job creation, promoting economic growth, improving healthcare, increasing research and development, and gaining international recognition. By leveraging these advantages, CROs can enhance their service offerings, including:
- Feasibility and selection of research locations
- Review and feedback on study documents
- Setup
- Comprehensive reporting on study status and adverse events
Key Criteria for Selecting an Experienced Medtech CRO
When selecting an experienced medtech CRO in the Dominican Republic, consider the following key criteria:
- Regulatory Knowledge: Ensure that the CRO has a comprehensive understanding of local regulations and guidelines governing clinical studies. Their ability to navigate these regulations is crucial for timely approvals and compliance, especially in complex environments like Colombia, where experts like Katherine Ruiz have successfully advised manufacturers on market clearance.
- Experience and Track Record: Evaluate the CRO's previous experience in conducting medtech trials, specifically in the Dominican Republic. A proven track record of successful studies, such as Early-Feasibility, First-In-Human, and Pivotal Studies managed by bioaccess®, indicates their capability to manage projects effectively.
- Team Expertise: Assess the qualifications and experience of the CRO's team members. Seek experts with a robust background in medtech research, including operational procedures, data management, and Regulatory Affairs, similar to the proficiency provided by bioaccess® with over 20 years in the field.
- Patient Recruitment Strategies: Investigate the CRO's strategies for patient recruitment and retention. Effective recruitment is crucial for the success of clinical studies, and a CRO with established networks and relationships in the local healthcare system will have an advantage. This established network is crucial for ensuring timely patient enrollment and retention throughout the study.
- Quality Assurance Processes: Inquire about the CRO's quality assurance and monitoring practices. Robust quality control measures ensure that the study adheres to high standards, safeguarding the integrity of the data collected, as emphasized by bioaccess®'s comprehensive project management practices that are flexible and customized to meet specific study needs.
- Communication and Collaboration: Strong communication skills and a collaborative approach are essential for a successful partnership. Ensure that the CRO is responsive and transparent in their communication, facilitating a smooth working relationship.
By focusing on these criteria, potential clients can make informed decisions when selecting a CRO that aligns with their research objectives and can effectively manage the complexities of conducting medtech studies in the Dominican Republic.
Evaluating the CRO's Capabilities and Resources
When assessing a CRO's capabilities and resources, especially for medical device studies, consider the following aspects:
- Technological Infrastructure: Review the CRO's technological capabilities, including data management systems, electronic data capture (EDC) tools, and software for monitoring and reporting. Advanced technology can enhance data accuracy and streamline testing processes.
- Staffing and Expertise: Assess the size and expertise of the CRO's team. A well-staffed CRO like bioaccess®, with over 20 years of experience in Medtech, can better handle the complexities of research studies and provide valuable insights throughout the investigation.
- Site Management: Assess the CRO's experience in managing clinical research sites. Bioaccess® specializes in feasibility and selection of research sites and principal investigators (PI), ensuring compliance with protocols—crucial for the success of multicenter trials.
- Types of Studies: Bioaccess® has expertise in managing a variety of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), which are essential for a comprehensive evaluation of their capabilities.
- Patient Engagement Strategies: Inquire about the CRO's approach to patient engagement and retention. Effective strategies can significantly influence patient recruitment and overall study success.
- Regulatory Affairs Support: Ensure that the CRO has a dedicated regulatory affairs team that can navigate local and international regulations. Bioaccess® is approved as a business service provider for U.S. medical device companies in Colombia, assisting in reducing risks related to compliance.
By thoroughly assessing these capabilities, clients can choose a CRO that is not only experienced but also well-prepared to manage the specific requirements of their medtech research.
Assessing Financial Stability and Transparency
When evaluating the financial stability and transparency of a Contract Research Organization (CRO), it is essential to consider several key factors:
- Financial Health: Request detailed financial statements or reports to assess the CRO's financial health. A stable financial background is crucial, as it indicates the CRO’s capacity to sustain operations and support ongoing projects effectively.
- Budgeting Practices: Inquire about the CRO's budgeting methodologies, such as activity-based costing or zero-based budgeting, and their ability to adhere to agreed-upon budgets. Grasping how they handle expenses is crucial to preventing unforeseen financial issues during research studies. Current trends suggest that CROs with strong budgeting practices, like those using the Planning Suite by IQVIA, can manage the challenges of various research environments more efficiently while precisely forecasting study timelines and performance.
- Transparency in Pricing: Ensure that the CRO offers clear and detailed pricing structures. Transparency in costs is fundamental for avoiding hidden fees, which in turn facilitates better financial planning and trust in the CRO's operations. The CRO should provide a breakdown of costs associated with each phase of the study to enhance clarity.
- Insurance and Liability Coverage: Confirm that the CRO holds suitable insurance and liability coverage to protect against potential risks related to research studies. This is especially significant given the varied settings in which assessments are carried out.
Moreover, it is important to mention that the CRO's abilities encompass feasibility evaluations, site selection, compliance reviews, setup, and project management—all vital for efficient research execution. John Myklusch, a seasoned Exit Planner and Chief Financial Officer, brings over 20 years of experience in financial strategy, mergers and acquisitions, and risk management, further enhancing the CRO's ability to maintain financial health and transparency.
Moreover, IQVIA Technologies is hosting a webinar centered on budget forecasting and payments for studies, which tackles the intricacies of conducting research across various nations and emphasizes the significance of prompt payments. By focusing on these financial considerations, clients are better positioned to mitigate risks and select a CRO that not only demonstrates capability but also maintains financial stability and transparency in its operations. As demonstrated in the case analysis titled 'The Impact of Research Payments on Site-Sponsor Relationships,' effective management of research payments can significantly improve site-sponsor relationships, highlighting the significance of these evaluations for successful collaborations.
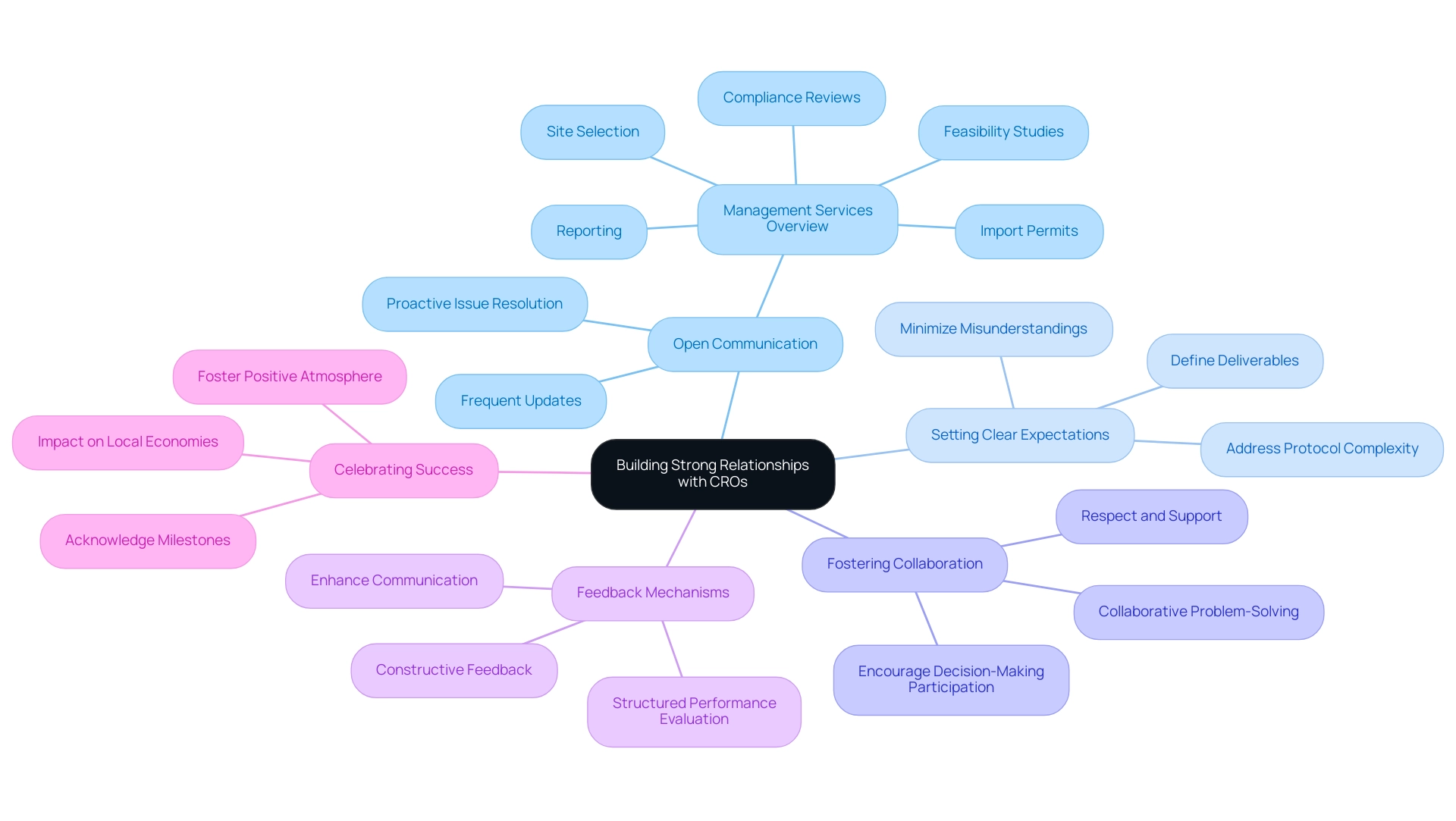
Building Strong Relationships with CROs
Establishing strong connections with Contract Research Organizations (CROs) is crucial for attaining favorable research results. Here are several strategies to consider:
- Open Communication: Establishing open lines of communication from the outset is crucial. Frequent updates and discussions enable proactive issue resolution and assist in sustaining project momentum, especially regarding the extensive management services that bioaccess® provides, encompassing feasibility studies, site selection, setup, compliance reviews, import permits, and reporting.
- Setting Clear Expectations: Clearly defining expectations and deliverables at the beginning of the partnership is vital. This clarity minimizes misunderstandings and ensures alignment between both parties, particularly important given that over 50% of sites express frustration over the complexity of protocols imposed by sponsors. By addressing these complexities upfront, both CROs and sponsors can work more effectively together, especially in navigating the regulatory landscape governed by INVIMA, Colombia's National Food and Drug Surveillance Institute.
- Fostering Collaboration: Encourage a collaborative environment where your team and the CRO's team actively participate in decision-making processes. Collaborative problem-solving not only strengthens partnerships but also enhances efficiency and inclusivity, which aligns with the FDA's emphasis on diversity in clinical research. As Denise N Bronner articulates, "Ultimately, building stronger ties between CROs and sites is not just about improving efficiency — it is about fostering a collaborative environment where all parties feel respected, supported, and aligned toward the same goal: delivering lifesaving treatments to diverse patient populations."
- Feedback Mechanisms: Implement structured feedback mechanisms to evaluate the CRO's performance. Providing constructive feedback can significantly improve the working relationship and lead to more successful future collaborations. The case study titled A Path Forward for Stronger CRO-Site Collaborations highlights that effective feedback not only enhances communication but also strengthens the overall partnership, ultimately improving patient recruitment from underrepresented populations.
- Celebrating Success: Acknowledge and celebrate milestones and achievements throughout the process. Recognizing successes fosters a positive atmosphere and reinforces the partnership's value, contributing to the overall impact on local economies through job creation and healthcare improvement.
By employing these strategies, clients can cultivate meaningful relationships with CROs, leading to not only successful clinical trial outcomes but also a mutually beneficial partnership that prioritizes inclusivity and efficiency, ultimately driving forward innovation and excellence in Medtech clinical research.
Conclusion
The medtech clinical trial landscape in the Dominican Republic presents a wealth of opportunities for international sponsors, driven by a favorable regulatory environment and a diverse participant pool. As the country gears up for approximately 15 clinical trials in 2024, it is vital for Contract Research Organizations (CROs) to navigate this evolving terrain effectively. Understanding local regulations, engaging with communities, and forming strategic partnerships are essential components for success in this promising market.
CROs must prioritize regulatory compliance and demonstrate expertise in local guidelines to ensure timely approvals and maintain research integrity. Additionally, fostering community engagement not only enhances transparency but also contributes to local economic growth and improved healthcare outcomes. By focusing on these aspects, CROs can create a robust framework for conducting successful medtech studies.
Moreover, selecting the right CRO involves evaluating their experience, communication practices, financial stability, and technological capabilities. A strong partnership built on open communication, collaboration, and mutual respect can significantly enhance trial efficiency and patient recruitment. As the Dominican Republic continues to emerge as a key player in the global medtech sector, leveraging these insights will empower CROs to maximize their impact and drive forward the future of healthcare innovation in the region.
Frequently Asked Questions
Why is the Dominican Republic becoming attractive for medtech research?
The Dominican Republic is becoming attractive for medtech research due to its favorable regulatory environment, a strong pool of qualified research participants, and recent governmental reforms that streamline approval processes for clinical studies.
How many clinical studies are projected to be conducted in the Dominican Republic in 2024?
Approximately 15 clinical studies are projected to be conducted in the Dominican Republic in 2024.
What factors enhance the feasibility of conducting medtech studies in the Dominican Republic?
The presence of well-established healthcare facilities and a diverse patient demographic enhance the feasibility of conducting medtech studies in the Dominican Republic.
What should Contract Research Organizations (CROs) understand to navigate the medtech research landscape effectively?
CROs should understand the changing regulations and guidelines governing medical studies in the Dominican Republic to ensure compliance and maintain research integrity.
What is the significance of skilled proposal writing in securing funding for clinical trials?
Skilled proposal writing is crucial for securing funding for clinical trials, as emphasized by a participant from URC7, who noted that the challenge lies in finding someone who can write the proposal.
How does community engagement impact medtech research in the Dominican Republic?
Involving local communities in the research process is essential for transparency and can contribute to job creation, economic growth, and improved healthcare, despite some resistance due to concerns about community capabilities.
What are some operational benefits that CROs can leverage in the Dominican Republic?
CROs can leverage operational benefits such as feasibility and selection of research locations, review and feedback on study documents, setup, and comprehensive reporting on study status and adverse events.
What key criteria should be considered when selecting a medtech CRO in the Dominican Republic?
Key criteria include regulatory knowledge, experience and track record, team expertise, patient recruitment strategies, quality assurance processes, and communication and collaboration skills.
What aspects should be assessed regarding a CRO's capabilities and resources for medical device studies?
Aspects to assess include technological infrastructure, staffing and expertise, site management, types of studies managed, patient engagement strategies, and regulatory affairs support.
What financial factors are important when evaluating a CRO's stability and transparency?
Important financial factors include financial health, budgeting practices, transparency in pricing, and insurance and liability coverage.
How can clients establish strong connections with CROs to achieve favorable research results?
Clients can establish strong connections by maintaining open communication, setting clear expectations, fostering collaboration, implementing feedback mechanisms, and celebrating successes throughout the research process.

