Overview
Choosing the right clinical trials suppliers involves careful consideration of factors such as regulatory compliance, supplier experience, quality assurance processes, and logistical capabilities. The article outlines a step-by-step guide emphasizing the importance of these criteria in ensuring successful research outcomes, highlighting how a thorough evaluation of potential suppliers can significantly impact the efficiency and integrity of clinical trials.
Introduction
In the intricate world of clinical research, the role of suppliers is often overlooked yet undeniably crucial. These entities, ranging from pharmaceutical companies to logistics providers, form the backbone of successful clinical trials, ensuring that essential materials and services are readily available. As the demand for high-quality clinical trial supplies surges—driven by the increasing prevalence of chronic diseases and the competitive landscape of nearly 50,000 pharmaceutical and biotech companies worldwide—understanding how to select and manage these suppliers becomes paramount. This article delves into the multifaceted aspects of clinical trial suppliers, from key selection criteria to effective procurement processes, while also highlighting the significance of logistics, inventory management, and sustainability in enhancing trial outcomes. Amidst the evolving trends and innovations, the focus remains on fostering reliable partnerships that not only drive research success but also contribute to the advancement of healthcare.
Understanding Clinical Trial Suppliers and Their Importance
Providers of research studies, also known as clinical trials suppliers, play a crucial role in offering the essential materials, services, and support required for the success of medical investigations. This varied group encompasses pharmaceutical firms, laboratories, equipment producers, and clinical trials suppliers, all of which play a crucial role in the complex network that supports research studies. With almost 50,000 pharmaceutical and biotech firms worldwide, the environment is extensive and growing more competitive, highlighting the essential requirement for clinical trials suppliers to meet the rising needs of research studies.
Significantly, partnerships such as the one between bioaccess™ and Caribbean Health Group establish Barranquilla as a top location for research studies in Latin America, backed by Colombia's Minister of Health, boosting local economies through job creation and healthcare enhancement.
The significance of clinical trials suppliers cannot be underestimated, as they directly affect the quality, efficiency, and adherence of research studies. By ensuring investigators have access to necessary resources—ranging from investigational drugs to laboratory supplies—clinical trials suppliers play a crucial role in facilitating studies that adhere to stringent regulatory standards. Thorough research study management services, including:
- feasibility assessments
- site selection
- compliance evaluations
- study setup
- project oversight
- reporting
are vital for success.
bioaccess™ and Caribbean Health Group offer essential services such as study setup and project management, ensuring compliance with Good Manufacturing Practices (GMP) and alignment with study objectives. This is further illustrated by the role of data management providers for medical research, who specialize in gathering, processing, and storing information from medical studies, ensuring precision and adherence to regulatory standards.
As the burden of chronic illnesses continues to rise globally, the demand for high-quality supplies from clinical trials suppliers for medical research will only increase, making the selection of reliable providers a critical consideration for researchers.
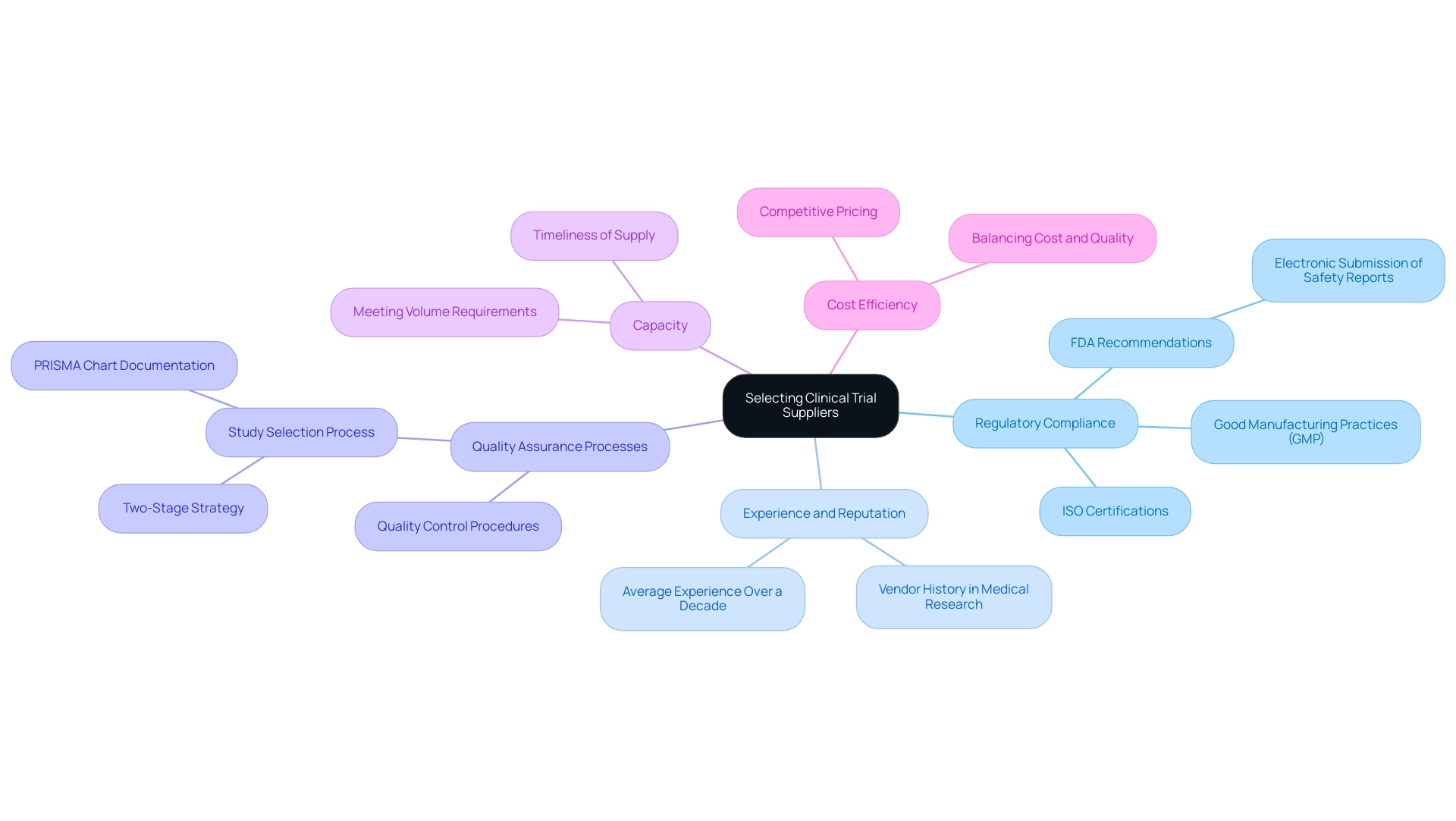
Key Factors to Consider When Selecting Clinical Trial Suppliers
When choosing clinical trials suppliers, several critical factors must be considered to ensure the success of your research. First and foremost is Regulatory Compliance. It's imperative to confirm that vendors comply with industry regulations and standards, including Good Manufacturing Practices (GMP) and International Organization for Standardization (ISO) certifications.
The FDA actively recommends electronic submission of safety reports, underscoring the importance of having a compliant supply chain that can facilitate rapid communication. This compliance is particularly essential considering the revenue from the cardiovascular diseases segment, which amounted to USD 1,198.7 million in 2023, emphasizing the financial stakes involved in choosing the appropriate partners. As Oommen John, a corresponding author, stated,
With this intention, we conducted a scoping review of the literature to identify regulatory documents that have guided trial investigators in trial data sharing.
This emphasizes the importance of comprehending regulatory frameworks.
Next, consider the experience and reputation of potential clinical trials suppliers. Assessing a vendor's history in medical research offers understanding of their dependability and skill. A provider with significant experience—averaging over a decade—can often maneuver the complexities of clinical studies more adeptly, which is a hallmark of bioaccess® as a leading organization among clinical trials suppliers facilitating medical device clinical studies in Latin America.
Quality Assurance Processes are another vital area of focus. Examine the vendor's quality control procedures to guarantee the integrity and reliability of the materials supplied during the experiment. This diligence is crucial as it directly affects the outcomes provided by clinical trials suppliers.
For instance, the study selection process in a recent case study involved a two-stage strategy that ensured transparency and adherence to specific inclusion and exclusion criteria, ultimately enhancing the quality of the selected clinical trials suppliers. Additionally, assess the capacity of the clinical trials suppliers to meet demand. It is essential to determine whether they can meet the volume and timing requirements specific to your research study, which can often be crucial to maintaining study timelines. This capacity is enhanced by bioaccess®'s dedication to information security in clinical trials suppliers, ensuring that all research study processes are protected against risks.
Bioaccess® employs reasonable security measures to prevent the loss, misuse, or unauthorized alteration of information, although it cannot guarantee absolute security, emphasizing the importance of selecting reliable clinical trials suppliers. Finally, while cost efficiency is a significant consideration, it should not overshadow quality. Competitive pricing can certainly influence vendor selection, but it is crucial to strike a balance between cost and the quality of the materials and services provided. By thoughtfully considering these factors, researchers can create a strong supplier network that fulfills the stringent requirements of their studies, backed by reliable partners like bioaccess®.
Navigating the Procurement Process in Clinical Trials
Navigating the procurement process in clinical studies with the help of clinical trials suppliers is a critical endeavor that requires meticulous planning and execution. Here are the essential steps involved:
-
Needs Assessment: Begin by identifying the specific supplies and services necessary for the experiment, including feasibility studies, site selection, and compliance reviews.
This foundational step ensures that all requirements are clearly defined.
-
Provider Evaluation: Utilize established criteria to shortlist potential providers.
Effective evaluation by clinical trials suppliers is crucial, as it can significantly influence the success of the trial. In 2024, the average time taken for vendor evaluation is projected to be streamlined through innovative practices, which can enhance overall efficiency.
-
Request for Proposals (RFP): Issue RFPs to chosen vendors, specifying your requirements and expectations clearly.
This process is pivotal for obtaining competitive bids from clinical trials suppliers and fostering transparent communication. Recent trends highlight that companies are refining their RFP processes to align with industry best practices, ensuring comprehensive proposals are received.
-
Negotiation: Enter discussions to finalize terms, pricing, and delivery schedules.
This stage is vital for establishing a mutually beneficial agreement.
As noted by Meghan Rexer, effective negotiation can enhance financial performance; low-touch planning can improve Return on Equity (ROE) by 2 to 4 percentage points and contribute an additional 1 to 3 percent to gross margins across various metrics.
-
Contracting: Once a vendor is selected, formalize the agreement through a contract that delineates responsibilities, timelines, and compliance requirements.
This step is essential for mitigating risks and ensuring accountability.
-
Order Placement: Finally, place orders with the supplier, ensuring that all specifications are communicated clearly to prevent misunderstandings.
By following these steps, research teams can optimize their procurement processes related to clinical trials suppliers and contribute to the overall success of their initiatives. In Colombia, the competitive benefits for first-in-human studies are notable, including cost efficiency, regulatory speed, high-quality healthcare, patient recruitment, and R&D tax incentives. These factors, combined with comprehensive CRO clinical study services that encompass all aspects of management—from feasibility studies to project coordination and reporting—enhance the potential for successful outcomes.
Specifically, effective experiment setup ensures that all logistical aspects are addressed, while robust project management guarantees that the study progresses smoothly and on schedule. Additionally, thorough reporting practices offer transparency and accountability throughout the process. As the healthcare research logistics market continues to develop, emphasized by Bomi Group's recent purchase by UPS to improve global healthcare logistics abilities, the significance of effective procurement practices cannot be overstated.
Furthermore, integrating unique insights from industry experts can significantly enhance the quality of procurement decisions.
Logistics and Transportation: Ensuring Timely Delivery of Supplies
Efficient logistics and transportation are essential to the success of supply management for studies, particularly when collaborating with clinical trials suppliers like bioaccess®, which has over 20 years of experience in the Medtech sector. As Kinjoll Dey emphasizes,
These transformations are crucial as the market is projected to grow from an estimated $10.42 billion in 2024, reaching $14.5 billion by 2032, with a compound annual growth rate of 3.74%.
This growth emphasizes the rising significance of prompt delivery in research studies, an element that clinical trials suppliers should prioritize, as it can greatly affect study results.
For instance, bioaccess® specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), all of which depend on robust logistics from clinical trials suppliers to ensure successful execution of the studies. The case study on safety analysis underscores the need for strong logistics provided by clinical trials suppliers to prevent treatment interruptions and study withdrawals, especially in light of disruptions caused by the COVID-19 pandemic. To ensure timely delivery, consider implementing the following strategies in collaboration with clinical trials suppliers:
-
Select Reliable Clinical Trials Suppliers:
Choose transportation providers with proven expertise in managing research materials for clinical trials to minimize risks associated with delays.
-
Plan for Contingencies:
Develop contingency plans for potential delays, such as alternative transportation routes or backup plans involving clinical trials suppliers, to mitigate disruptions.
-
Track Shipments:
Utilize advanced tracking systems to monitor shipments in real-time, which is essential for clinical trials suppliers to enable proactive issue management. For instance, successful tracking examples in medical research have demonstrated how clinical trials suppliers can facilitate timely interventions to resolve delivery issues before they impact trial outcomes.
-
Temperature Control:
Ensure that clinical trials suppliers can maintain the required conditions during transit for temperature-sensitive items, thereby safeguarding their integrity.
-
Documentation:
Prepare all necessary shipping and regulatory paperwork in advance to prevent customs delays, a frequent challenge faced by clinical trials suppliers in healthcare logistics. The news regarding missing data patterns after study unblinding also emphasizes the need for accurate documentation to prevent issues that can arise from delays. Considering that the cardiovascular diseases segment generated USD 982.2 million in 2020 and is anticipated to reach USD 1,198.7 million by 2023, the financial stakes highlight the significance of efficient logistics strategies provided by clinical trials suppliers in study management, as inadequate logistics can directly affect both study integrity and financial results.
By leveraging bioaccess®'s expertise, including feasibility and selection of research sites, compliance reviews, and project management, you ensure a streamlined process with clinical trials suppliers that enhances compliance and ultimately contributes to economic growth and healthcare improvements in local economies.
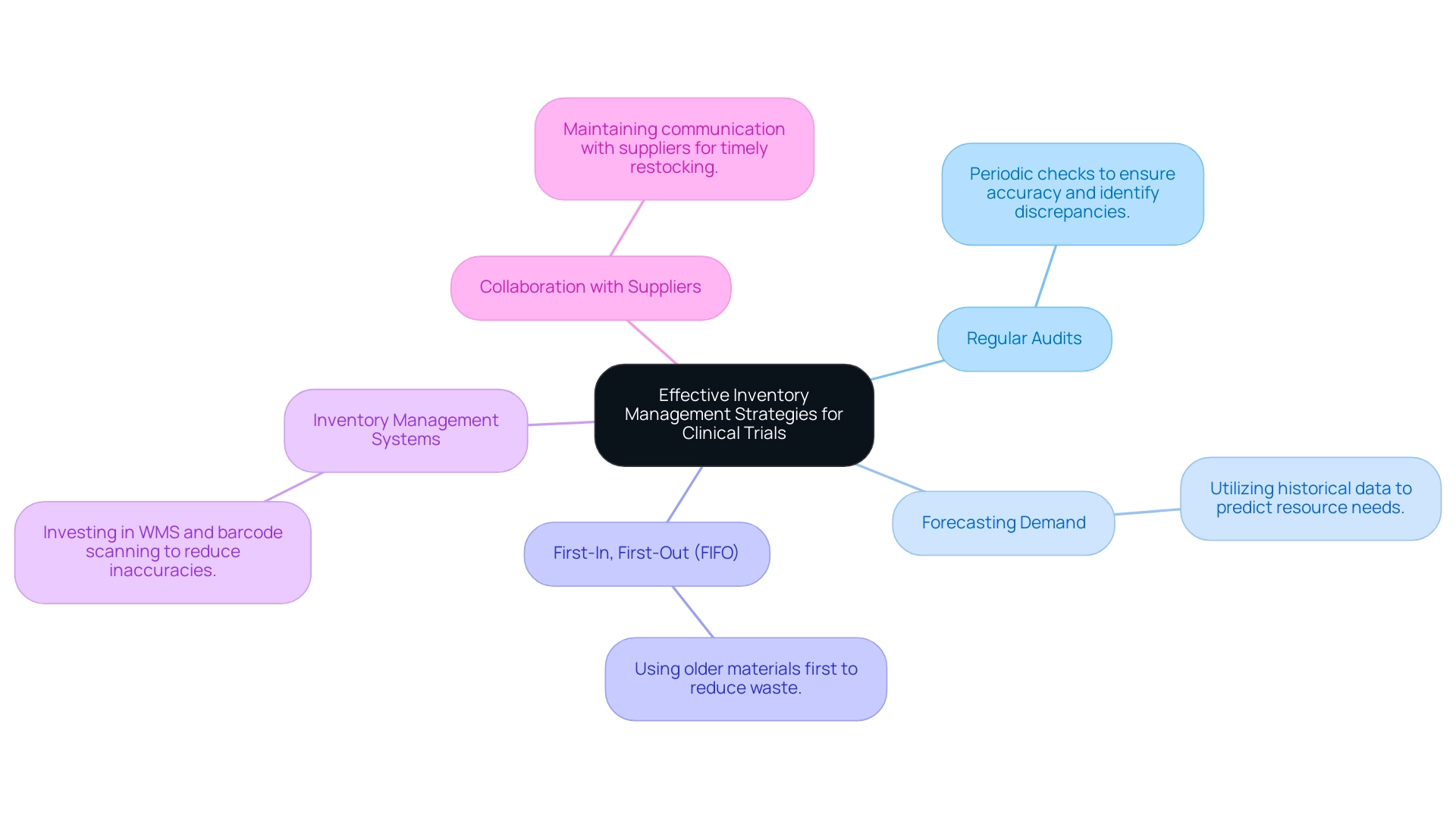
Effective Inventory Management Strategies for Clinical Trials
Efficient inventory management techniques are crucial for the success of clinical studies, particularly with the involvement of clinical trials suppliers in handling resources across various locations. Consider implementing these key practices:
-
Regular Audits: Periodic inventory audits are vital for ensuring accuracy and identifying discrepancies. Regular checks can help maintain integrity and prevent issues associated with overstocking or shortages.
-
Forecasting Demand: Utilize historical data alongside trial timelines to accurately predict resource needs, thereby minimizing the risk of shortages.
-
First-In, First-Out (FIFO): Adopt a FIFO approach to ensure that older materials are utilized before newer ones, effectively reducing waste and optimizing resource use.
-
Inventory Management Systems: Invest in advanced warehouse management systems (WMS) and barcode scanning technologies, which have been shown to reduce inaccuracies to just 5-10%. These tools can help track inventory levels, manage orders, and streamline replenishment processes.
-
Collaboration with Clinical Trials Suppliers: Foster open lines of communication with clinical trials suppliers to discuss inventory levels and anticipated needs, ensuring timely restocking. As Oliver Munro observes,
Global warehousing property costs grew 10.1% in 2023,
which underscores the importance of efficient inventory management.
Furthermore, the case study on handling missing data during COVID-19 highlights how crucial it is to collect relevant data to avoid missing not at random (MNAR) assumptions. This connection demonstrates that efficient inventory management methods not only preserve resources but also protect the integrity of research results, emphasizing the necessity for thorough strategies in research studies. Notably, this guidance is supported by the expertise of authors associated with the Rutgers Center of Operations Research and the Department of Supply Chain Management & Marketing Sciences at Rutgers University.
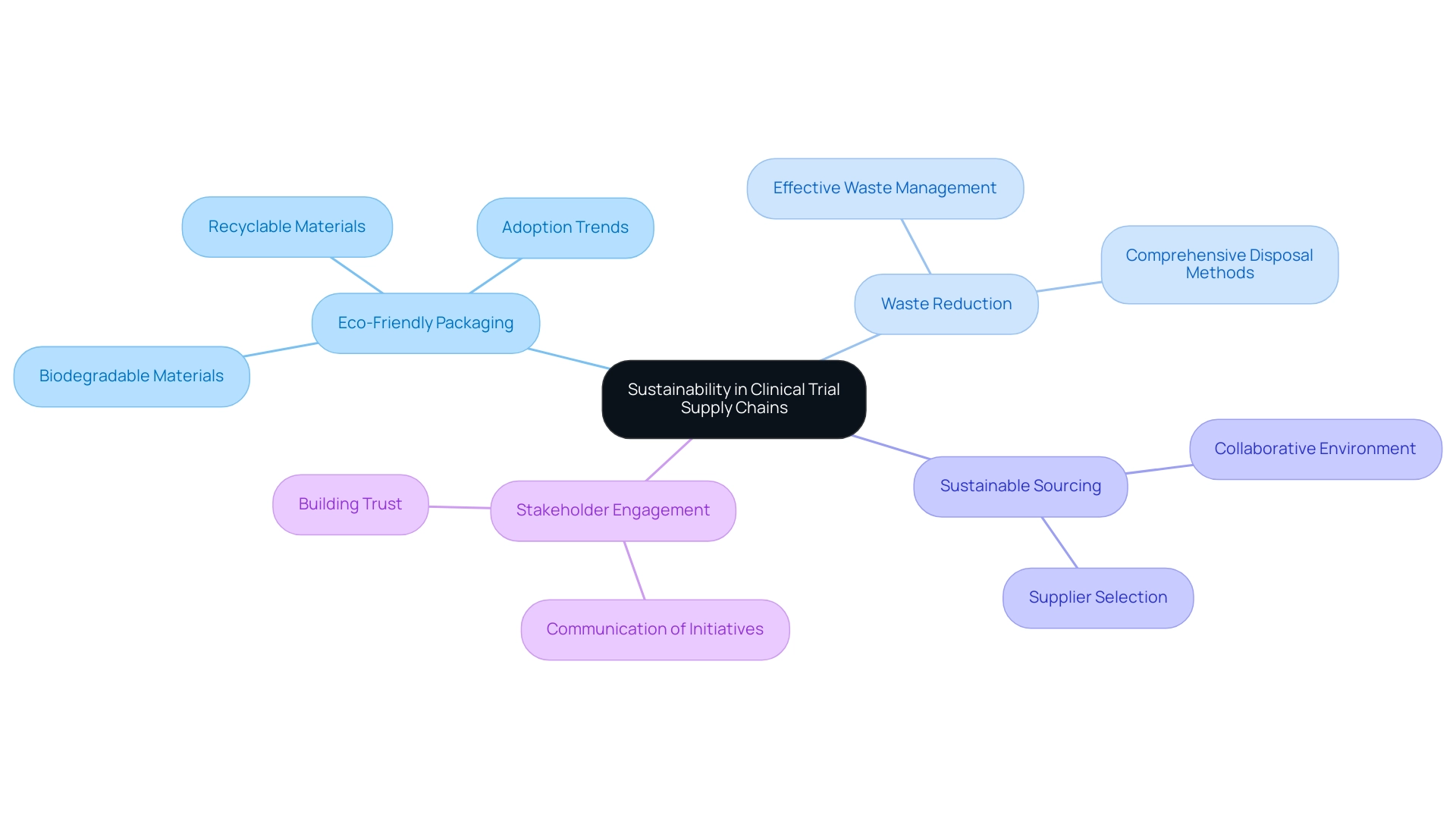
Sustainability in Clinical Trial Supply Chains: Best Practices
Sustainability is progressively acknowledged as an essential element in the management of research logistics by clinical trials suppliers. Implementing best practices can significantly enhance operational efficiency while also addressing environmental concerns. Key strategies include:
-
Eco-Friendly Packaging: Utilizing biodegradable or recyclable materials for packaging supplies is paramount.
Recent studies indicate that a growing percentage of clinical trials suppliers are adopting eco-friendly packaging solutions, reflecting a shift towards environmental responsibility.
-
Waste Reduction: Effective waste management strategies should be implemented to minimize waste throughout the process, including comprehensive disposal methods for any unused materials.
-
Sustainable Sourcing: Selecting clinical trials suppliers who prioritize sustainability not only supports eco-friendly practices but also fosters a collaborative environment focused on shared values.
Engaging clinical trials suppliers that utilize energy-efficient transportation methods can greatly reduce the carbon footprint associated with research trials.
- Stakeholder Engagement: It is essential to communicate sustainability initiatives to stakeholders, including sponsors and regulatory bodies. This transparency enhances accountability and builds trust in the clinical research process.
Significantly, 90% of a pharmaceutical company’s emissions are Scope 3 emissions, produced by their chains, highlighting the need for sustainable practices. In 2023, ICON exemplified this commitment by conducting 120 supplier audits and actively engaging suppliers to advance their Environmental, Social, and Governance (ESG) efforts, resulting in formal emissions reduction plans with partners in the largest contributing areas. Furthermore, the interaction effects of sustainability inhibitors emphasize the significance of managing these barriers to improve the effectiveness of sustainable chain strategies.
Such efforts not only contribute to the sustainability goals of the pharmaceutical sector but also align with the increasing demand for eco-friendly practices within the industry.
Future Trends and Innovations in Clinical Trial Supplies
The landscape of clinical trials suppliers is on the brink of substantial transformation, driven by several pivotal trends. First, the rise of Digital Supply Chain Management is transforming how organizations track and manage resources in real-time, allowing for enhanced visibility and efficiency. This shift has been particularly crucial as organizations evaluate their operations for improvement opportunities, especially in light of the disruptions caused by the Covid-19 pandemic, which highlighted the need for more resilient logistics chains.
The case study on decentralized manufacturing illustrates how this approach not only enhances resource security but also accelerates access to sensitive materials such as biologics, thereby reducing regulatory delays. Significantly, facilities that embraced decentralized manufacturing reported a 30% decrease in time to market for essential materials, highlighting the concrete advantages of this model.
Next, clinical trials suppliers are transforming the delivery and management of resources in medical studies through Telehealth Integration. The incorporation of telehealth solutions facilitates direct engagement with patients, streamlining logistics and enhancing the overall experience. This integration is gaining more significance as clinical studies aim to be more patient-focused, ensuring that clinical trials suppliers provide resources effectively and customize them to individual patient requirements.
The implementation of AI and Machine Learning further improves these developments. These technologies enable sponsors to utilize predictive analytics, allowing them to make proactive decisions concerning logistics and enhance inventory management. This capacity for accurate forecasting significantly mitigates the risks associated with shortages and excesses.
Moreover, the exploration of Blockchain for Transparency is gaining traction, promising to enhance traceability and accountability within the distribution chain. For example, adopting blockchain solutions has enabled organizations to trace the origin of medical research materials, guaranteeing that each transaction is documented and reachable. This level of transparency builds trust among stakeholders and safeguards against potential disruptions, particularly in managing sensitive materials.
Finally, the focus on Patient-Centric Approaches is increasingly influencing the strategies of clinical trials suppliers. Innovations focused on enhancing patient experiences and results are becoming essential to the design and implementation of research studies. According to a report by the European Medicines Agency, these developments enable patients to access novel therapies more easily and quickly, as production facilities are located closer to them.
As we look towards 2024 and beyond, the integration of these digital tools, alongside the adoption of advanced technologies, will redefine the future of clinical trial supplies.
Conclusion
The intricate world of clinical trial suppliers is essential for the success of clinical research, as highlighted throughout this article. These suppliers, which include pharmaceutical companies, logistics providers, and laboratories, form the backbone of clinical trials by ensuring the availability of necessary materials and services. Key factors such as regulatory compliance, supplier experience, quality assurance, and effective inventory management emerge as critical considerations for researchers aiming to select reliable partners.
Navigating the procurement process meticulously and implementing robust logistics and inventory strategies are vital steps that contribute to the seamless execution of clinical trials. The importance of sustainability and innovative practices, such as digital supply chain management and telehealth integration, is also underscored, reflecting a shift towards more efficient and environmentally responsible operations.
Ultimately, fostering strong partnerships with trustworthy suppliers not only enhances trial outcomes but also propels advancements in healthcare. As the landscape of clinical trials continues to evolve, staying attuned to these trends and best practices will be crucial for researchers seeking to navigate this complex environment successfully. Emphasizing the significance of these collaborative efforts can pave the way for improved healthcare solutions and better patient outcomes in the future.
Frequently Asked Questions
What is the role of clinical trials suppliers in medical research?
Clinical trials suppliers provide essential materials, services, and support necessary for the success of medical investigations, including pharmaceutical firms, laboratories, and equipment producers. They ensure access to resources like investigational drugs and laboratory supplies, facilitating studies that comply with regulatory standards.
How do partnerships impact clinical trials?
Partnerships, such as between bioaccess™ and Caribbean Health Group, enhance research study locations, like Barranquilla in Latin America, and contribute to local economies by creating jobs and improving healthcare.
What services do clinical trials suppliers offer?
Clinical trials suppliers provide a range of services, including feasibility assessments, site selection, compliance evaluations, study setup, project oversight, and reporting, which are vital for the success of research studies.
Why is regulatory compliance important when selecting clinical trials suppliers?
Regulatory compliance ensures that suppliers adhere to industry standards, such as Good Manufacturing Practices (GMP) and ISO certifications, which are crucial for maintaining a compliant supply chain and facilitating rapid communication, especially in high-stakes areas like cardiovascular research.
What factors should be considered when choosing clinical trials suppliers?
Key factors include regulatory compliance, the experience and reputation of suppliers, quality assurance processes, capacity to meet demand, and cost efficiency balanced with quality.
How does the experience of a supplier influence clinical trials?
A supplier with significant experience, particularly over a decade, can navigate the complexities of clinical studies more effectively, enhancing the overall quality and reliability of the research.
What is the significance of quality assurance processes in clinical trials?
Quality assurance processes ensure the integrity and reliability of the materials supplied, directly affecting the outcomes of clinical trials.
How does bioaccess® ensure information security in clinical trials?
Bioaccess® employs reasonable security measures to protect research study processes against risks, although it cannot guarantee absolute security, highlighting the importance of selecting reliable suppliers.
Why is cost efficiency a consideration in selecting clinical trials suppliers?
While cost efficiency is important, it should not compromise the quality of materials and services provided. A balance between cost and quality is essential for successful research studies.




