Introduction
Navigating the regulatory landscape for clinical trials in Colombia presents both challenges and opportunities for researchers and organizations alike. With the National Institute for Food and Drug Surveillance (INVIMA) at the helm, understanding the intricacies of compliance and ethical considerations becomes paramount. As the authority responsible for overseeing clinical research, INVIMA’s established guidelines serve as a foundation for conducting safe and effective trials.
This article delves into the essential steps for engaging a Contract Research Organization (CRO), emphasizing the importance of regulatory knowledge, operational capabilities, and effective communication. By equipping stakeholders with the necessary insights, it aims to facilitate successful first-in-human studies and ensure adherence to the highest standards of clinical research.
Navigating Colombia's Regulatory Environment for Clinical Trials
Before involving a First-in-Human Studies CRO Colombia, it is crucial to understand the regulatory obligations that oversee medical studies in Colombia. The National Institute for Food and Drug Surveillance (INVIMA) is the main authority supervising research in the country, created under the Ministry of Health and Social Protection. A comprehensive understanding of the guidelines set by First-in-Human Studies CRO Colombia is crucial.
INVIMA is responsible for inspecting and supervising the marketing and manufacturing of health products, as well as ensuring compliance with established health standards. Notably, INVIMA has been classified as a Level 4 regional reference health authority by the Pan American Health Organization/World Health Organization, underscoring its competence in regulating health products efficiently. Within INVIMA, the Directorate for Medical Devices and other Technologies plays a crucial role in overseeing medical devices, ensuring that they meet safety and efficacy standards.
Here are the key steps to consider:
- Research INVIMA Guidelines: Stay updated with the latest regulations related to medical studies, specifically those that address First-in-Human Studies CRO Colombia. Resolution No. 2378 of 2008, which adopted Good Clinical Practices, is a critical reference point.
- Understand Ethical Considerations: Acquaint yourself with the ethical review processes, which necessitate obtaining approval from an ethics committee. Marta Faccini highlights that, "The primary aim of these regulations is to ensure the protection of the rights, safety, and well-being of the individuals involved in research, guaranteeing compliance with ethical and scientific standards." This underscores the significance of ethical considerations at every stage of the trial process.
- Compliance with Local Laws: Ensure that your research adheres to local regulations concerning patient consent, data protection, and the reporting of adverse events, which are fundamental to maintaining the integrity of clinical research. Additionally, research centers are required to implement Quality and Risk Management programs to ensure compliance with these regulations.
- Engage with Local Experts: Collaborate with local regulatory experts like Katherine Ruiz, who has extensive experience in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia. Her insights can be invaluable in navigating the complexities associated with compliance. It's also advantageous to examine case analyses related to INVIMA's guidelines, as these can offer practical examples of successful navigation through the regulatory landscape.
- Comprehensive Clinical Trial Management: Ensure that your First-in-Human Studies CRO Colombia is capable of handling all aspects of trial setup, including project management and monitoring. This involves acquiring required import permits for investigational devices and ensuring that all documentation complies with local requirements.
By thoroughly understanding the regulatory landscape, you can more effectively evaluate potential CROs for their ability to manage compliance and uphold the ethical standards necessary for high-quality clinical research.
Key Criteria for Choosing the Right CRO for First-in-Human Studies
When selecting a First-in-Human Studies CRO Colombia, consider the following key criteria:
- Experience in First-in-Human Studies CRO Colombia: Seek CRO Colombia with demonstrated proficiency in executing first-in-human studies, as this necessitates specialized knowledge and protocols. For instance, bioaccess™ has successfully partnered with GlobalCare Clinical Trials, recognized as a First-in-Human Studies CRO Colombia, to enhance ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and a 95% retention rate, which underscores its effectiveness as a CRO.
- Regulatory Knowledge: Ensure the CRO is well-versed in Colombian regulations and has a track record of successful submissions to INVIMA. The backing from Colombia's Minister of Health for trial initiatives enhances the region's appeal for First-in-Human Studies CRO Colombia, strengthening the reliability of partnerships in the area.
- Quality Assurance Processes: Evaluate the CRO's quality management systems to ensure rigorous adherence to Good Clinical Practice (GCP).
- Site Management Capabilities: Evaluate their ability to manage research sites effectively, including patient recruitment and retention strategies. The collaboration with LATAM CRO Experts and local institutions strengthens these capabilities.
- Collaborative Approach: Select a CRO that prioritizes collaboration and communication, as strong partnerships can improve project success. Leaders such as Dushyanth Surakanti of Sparta Biomedical and Dr. John B. Simpson of Avinger highlight the significance of effective collaboration in their research experiences conducted with First-in-Human Studies CRO Colombia.
- Technological Infrastructure: Investigate the CRO's use of technology for data management and monitoring, which can streamline processes and improve data integrity.
By focusing on these criteria, you can select a CRO that aligns with your research objectives and enhances the likelihood of successful outcomes.
Evaluating the CRO's Track Record and Reputation
Assessing a Contract Research Organization's (CRO) history and standing is essential for guaranteeing the success of your trials, particularly with First-in-Human Studies CRO Colombia and in early-feasibility environments. Here are key steps to undertake:
-
Request References: Actively seek references from former clients. These insights can provide a clear understanding of the CRO's performance, client satisfaction, and any challenges faced during their partnership. According to recent statistics, CROs with a strong reputation often report higher client engagement, as evidenced by conversion rates averaging 3.68% globally.
-
Review Case Examples: Examine case examples that highlight the expertise of the First-in-Human Studies CRO Colombia in conducting first-in-human trials. For instance, bioaccess® has successfully supported Avantec Vascular in conducting its first-in-human trial for an innovative vascular device through the First-in-Human Studies CRO Colombia in Latin America, showcasing their ability to navigate regulatory challenges effectively. Furthermore, ReGelTec's Early Feasibility Study on HYDRAFIL™ in Colombia highlights the expertise of First-in-Human Studies CRO Colombia in managing complex evaluations, emphasizing compliance and successful outcomes.
-
Check Regulatory Compliance History: Delve into any historical regulatory violations or compliance issues that the CRO may have encountered. A clean compliance record is indicative of a commitment to quality control and adherence to industry standards. As highlighted in industry reviews, CROs with fewer compliance issues tend to have better client satisfaction ratings.
-
Consult Industry Reviews: Utilize reputable online platforms and industry forums to gather reviews and testimonials regarding the CRO’s services. This feedback can reveal the organization’s strengths and weaknesses from the perspective of peers in the field. A survey conducted in 2023 found that CROs frequently mentioned in positive reviews had an average conversion rate of 2.8% in the Netherlands, reflecting their effectiveness in client engagement.
-
Assess Longevity and Stability: Take into account the CRO's tenure in the industry. With more than 20 years of experience in Medtech, bioaccess® exhibits reliability, stability, and extensive expertise, all of which are essential for intricate research studies. As industry expert Dr. Jane Smith states, "A CRO's longevity often reflects its ability to adapt and thrive within evolving regulatory landscapes."
By thoroughly evaluating a CRO's track record through these methods, particularly considering their specialized services like feasibility analysis, site selection, project setup, project management, and reporting as demonstrated by bioaccess®, you position yourself to make a well-informed choice that aligns with the objectives and regulatory requirements of your research.
Understanding the CRO's Operational Capabilities
When assessing the operational capabilities of a Contract Research Organization (CRO) for your clinical evaluations, especially in the context of early-feasibility and first-in-human investigations in Latin America, it is crucial to concentrate on several key aspects:
- Project Management Expertise: Scrutinize the CRO’s project management processes. This includes assessing their methodologies for timeline adherence, resource allocation efficiency, and risk management strategies that mitigate potential challenges during the trial. bioaccess® exemplifies effective project management through its comprehensive service offerings that guide assessments from feasibility to post-market follow-up, ensuring compliance and fostering long-term partnerships with clients.
- Staff Qualifications: Investigate the qualifications and experience of the research team assigned to your project. It is crucial to ensure that team members possess relevant expertise, such as Clinical Data Managers, Medical Monitors, Biostatisticians, and Regulatory experts, to effectively support your project. bioaccess® boasts a team with over 20 years of experience in Medtech, ensuring that your study is managed by seasoned professionals.
- Patient Recruitment Strategies: Gain insight into the CRO's approach to patient recruitment. This encompasses their established networks and innovative strategies for engaging potential participants, which are vital for the success of your study. Notably, bioaccess® utilizes targeted outreach and digital engagement to enhance recruitment efforts, significantly impacting study timelines and outcomes.
- Data Management Systems: Evaluate the CRO's data management capabilities, particularly their electronic data capture (EDC) systems and robust data security measures. Strong data management is pivotal for maintaining data integrity and compliance. bioaccess® ensures rigorous data management practices that comply with local regulations and international standards.
- Monitoring and Reporting Practices: Review the CRO’s monitoring and reporting procedures throughout the study. Effective monitoring ensures adherence to regulatory standards and facilitates timely communication regarding study progress and any emerging issues. With bioaccess®, you can expect thorough project management and transparent reporting, keeping you informed every step of the way.
- Regulatory Navigation: Understand how the CRO navigates the regulatory environments pertinent to your research. bioaccess® is skilled at navigating the intricacies of regulatory adherence, encompassing the evaluation and input on research documents, setup of experiments, and acquiring essential import permits for investigational devices.
By comprehensively grasping these operational abilities, you can choose a CRO partner like bioaccess® that is not only well-prepared to oversee your First-in-Human Studies CRO Colombia but also in harmony with the strategic objectives of your research initiatives.
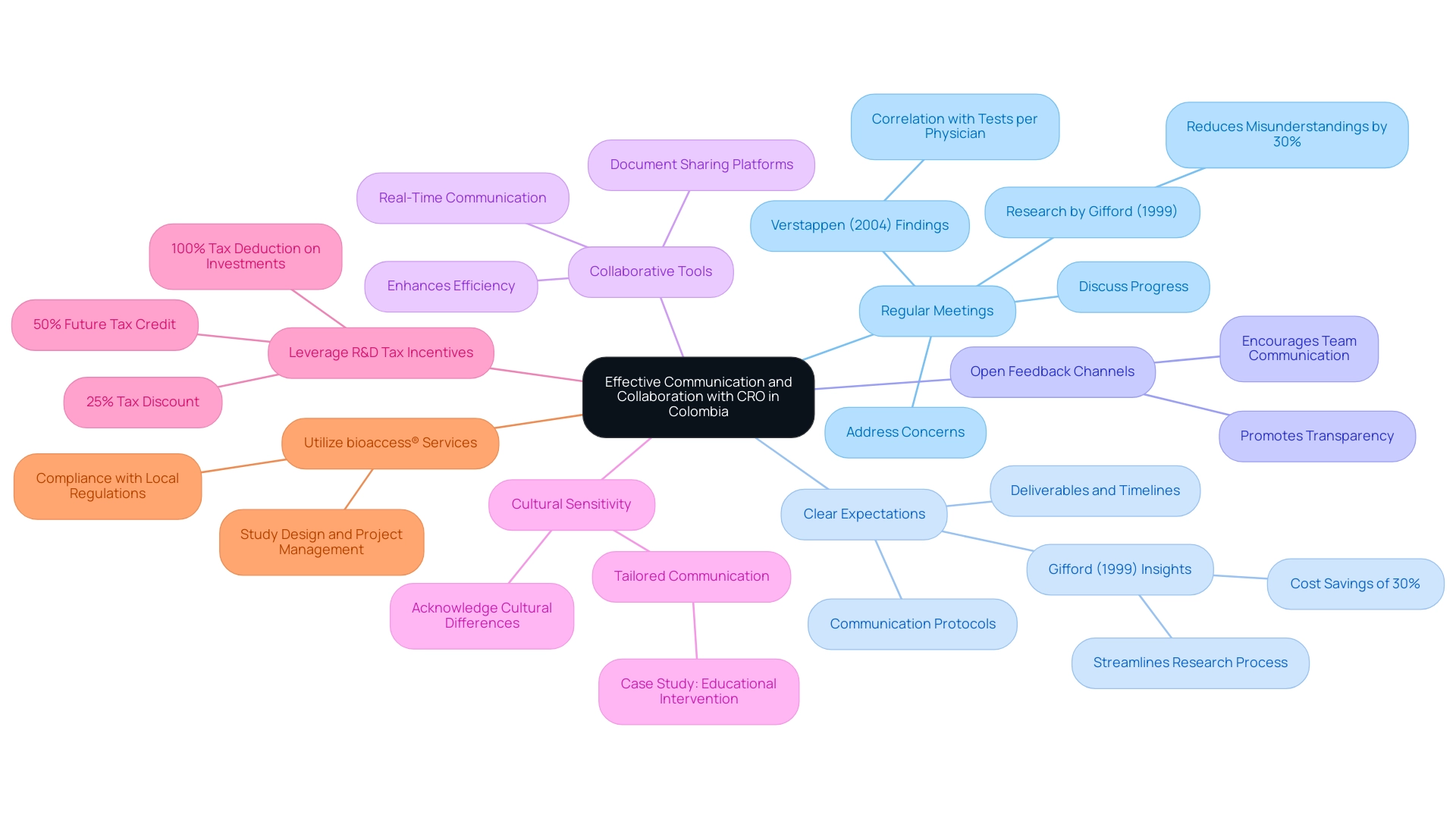
Establishing Clear Communication and Collaboration
To facilitate effective communication and foster collaboration with your chosen Contract Research Organization (CRO) in Colombia, consider implementing the following best practices:
-
Regular Meetings: Scheduling regular meetings is crucial for discussing progress, addressing concerns, and making necessary adjustments to the study plan. Research by Gifford (1999) demonstrated that effective communication can reduce misunderstandings by up to 30%, which is vital for enhancing the overall success of health studies.
Additionally, Verstappen (2004) found that the mean number of tests per physician was positively correlated with the frequency of communication, further supporting the importance of regular interactions.
-
Clear Expectations: Establishing clear expectations regarding deliverables, timelines, and communication protocols is essential to prevent misunderstandings. As highlighted in the work of Gifford (1999), clarity in communication can significantly streamline the research process, especially in the context of Colombia, where navigating regulatory environments can be complex yet beneficial due to cost savings of over 30% compared to North America or Western Europe.
-
Open Feedback Channels: Creating open channels for feedback promotes transparency and enables prompt resolution of issues. This approach encourages team members to voice concerns without hesitation, ultimately contributing to a more responsive project environment, particularly crucial in a setting where high-quality healthcare and patient recruitment are pivotal.
-
Collaborative Tools: Leverage collaborative tools and platforms designed for real-time communication and document sharing, which can enhance coordination among team members.
Examples of such tools include project management software and secure messaging platforms, which have been shown to enhance efficiency in clinical collaborations. This is particularly important for managing the extensive documentation and compliance requirements in Colombia’s regulatory framework.
-
Cultural Sensitivity: Acknowledge cultural differences that may impact communication and collaboration, particularly in diverse settings like Colombia.
Being culturally sensitive fosters stronger relationships, as demonstrated in the case study on educational interventions for preventive home visitors, which emphasized tailored communication to effectively engage community members. Grasping the local context can improve engagement and cooperation, vital for successful outcomes.
-
Leverage R&D Tax Incentives: Take advantage of Colombia's R&D tax incentives, which include a 100% tax deduction on investments in science, technology, and innovation projects, as well as a 25% tax discount and a 50% future tax credit.
Emphasizing these financial advantages can enhance the value proposition for conducting experiments in Colombia.
-
Utilize bioaccess® Services: Consider the specific services offered by bioaccess®, such as study design, project management, and setup, which can streamline the process and ensure compliance with local regulations. Their expertise in managing clinical studies can significantly enhance the efficiency and success of your research.
By prioritizing these communication and collaboration strategies, you can cultivate a productive partnership with the First-in-Human Studies CRO Colombia, thereby enhancing the likelihood of successful study outcomes. As noted by EJM, the value of consistent communication cannot be overstated:
We thank the trial managers based at the Nottingham Clinical Trials Unit who kindly provided data on investigator meetings held for the trials that they are responsible for.
This underscores the importance of regular engagement in building effective collaborative relationships, especially when leveraging Colombia's competitive advantages for First-in-Human Studies CRO Colombia and early-feasibility studies.
Conclusion
Thorough navigation of Colombia's regulatory landscape is critical for the success of clinical trials, particularly first-in-human studies. The National Institute for Food and Drug Surveillance (INVIMA) serves as the cornerstone of this process, providing essential guidelines that ensure compliance and uphold ethical standards. By understanding INVIMA's regulations, engaging local expertise, and maintaining rigorous ethical considerations, researchers can effectively manage the complexities associated with clinical trials in the region.
Selecting the right Contract Research Organization (CRO) is equally vital. Key criteria such as experience in first-in-human studies, regulatory knowledge, and operational capabilities play a pivotal role in this decision-making process. Evaluating a CRO's track record and reputation through references, case studies, and client reviews provides valuable insights into their effectiveness and reliability.
Furthermore, fostering clear communication and collaboration with the CRO enhances project outcomes and mitigates potential misunderstandings.
In conclusion, an informed approach that emphasizes regulatory compliance, strategic CRO selection, and effective communication is essential for conducting successful clinical trials in Colombia. By leveraging these best practices, stakeholders can not only navigate the regulatory environment with confidence but also contribute to the advancement of medical research and innovation in the region.




