Introduction
Chile stands at the forefront of clinical research, offering a dynamic landscape for conducting first-in-human studies. With a regulatory framework that emphasizes efficiency and ethical standards, the country has become an attractive destination for sponsors looking to accelerate their research timelines. This article delves into the multifaceted advantages of conducting clinical trials in Chile, highlighting the critical role of Contract Research Organizations (CROs) in navigating the regulatory landscape, ensuring compliance, and fostering successful collaborations.
By exploring the essential criteria for evaluating CROs, the importance of clear communication, and the assessment of post-study outcomes, stakeholders can make informed decisions that leverage Chile’s unique opportunities in the realm of clinical research.
Navigating the Regulatory Landscape for Clinical Trials in Chile
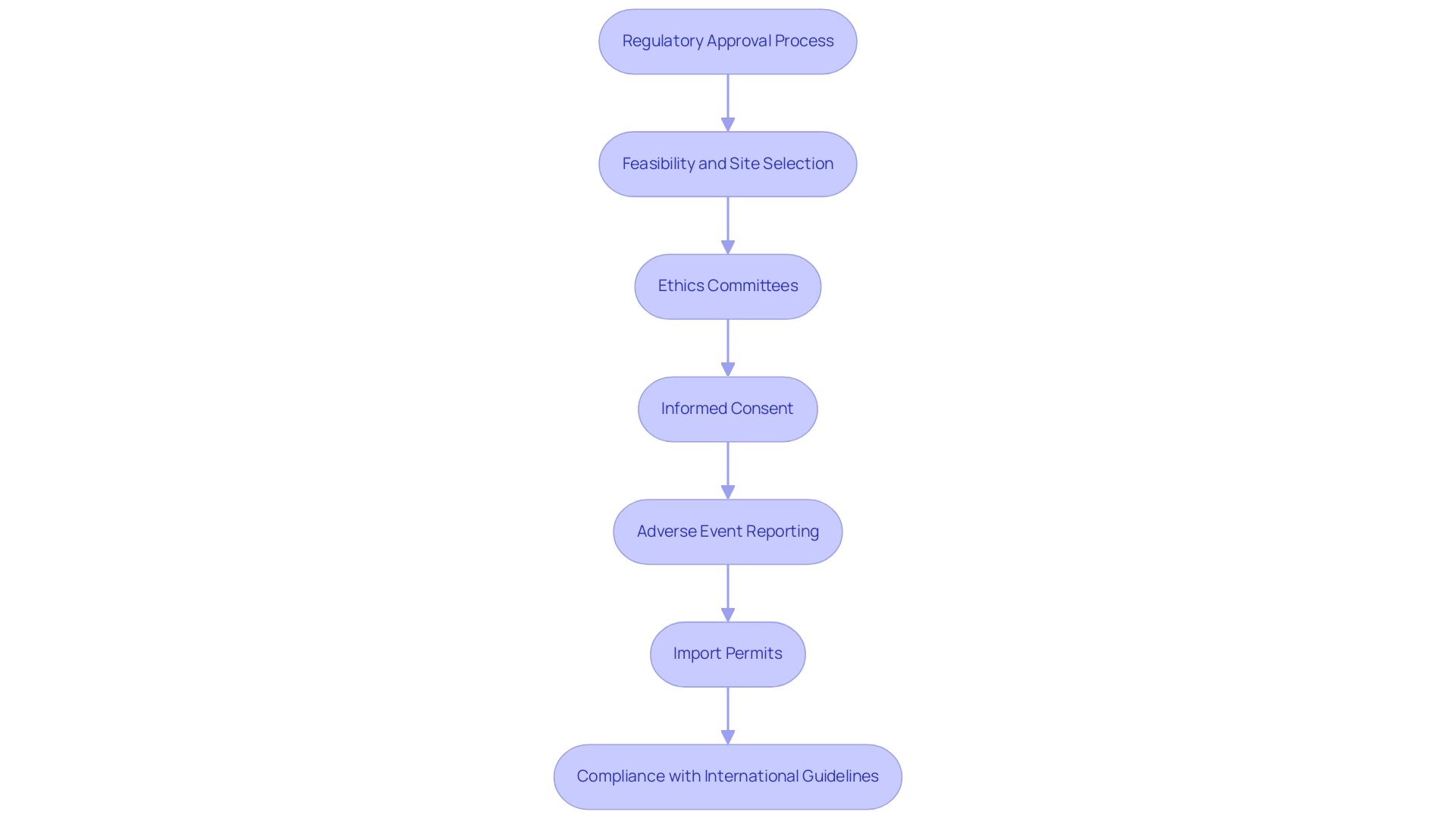
In Chile, research studies must adhere to the regulations established by the Instituto de Salud Publica (ISP) and the ethics committees that supervise research activities. It is essential to familiarize yourself with the following key components:
- Regulatory Approval Process: Before starting a clinical study, obtain approval from the ISP. This involves submitting a detailed protocol that outlines the study's objectives, methodology, and safety measures, reflecting the stringent compliance reviews emphasized by experts like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia.
- Feasibility and Site Selection: Consider the feasibility and choice of research locations and principal investigators (PIs), which are crucial for the success of the study. This aligns with bioaccess®'s comprehensive service capabilities in identifying suitable sites and qualified PIs.
- Ethics Committees: All medical studies must obtain ethical approval from an Institutional Review Board (IRB). This guarantees that the rights and welfare of participants are safeguarded during the study, a crucial element of bioaccess®'s extensive management services.
- Informed Consent: Ensure that the CRO has robust processes for obtaining informed consent from participants, which is a critical requirement in all research studies and aligns with the practices advocated by Juan Cuya, MD, who focuses on health technology and public health education at Universidad de Los Andes.
- Adverse Event Reporting: Familiarize yourself with the obligations for reporting adverse events and serious adverse events to regulatory authorities, a crucial component for maintaining trial integrity and safety, highlighting the importance of project management in research trials.
- Import Permits and Nationalization of Investigational Devices: Understand the requirements for obtaining import permits and nationalizing investigational devices, which are essential for compliance and operational success in research trials.
- Compliance with International Guidelines: Ensure that the CRO adheres to Good Clinical Practice (GCP) and other international guidelines, which are essential for the credibility of the research and the safety of participants.
By thoroughly understanding these regulatory aspects, you can better assess potential CROs and their capabilities in managing compliance effectively, reflecting the innovative and regulatory excellence that bioaccess® brings to clinical research in Latin America.
Why Choose Chile for First-in-Human Studies: Opportunities and Advantages
Chile has emerged as a prime location for first-in-human research, driven by several compelling advantages:
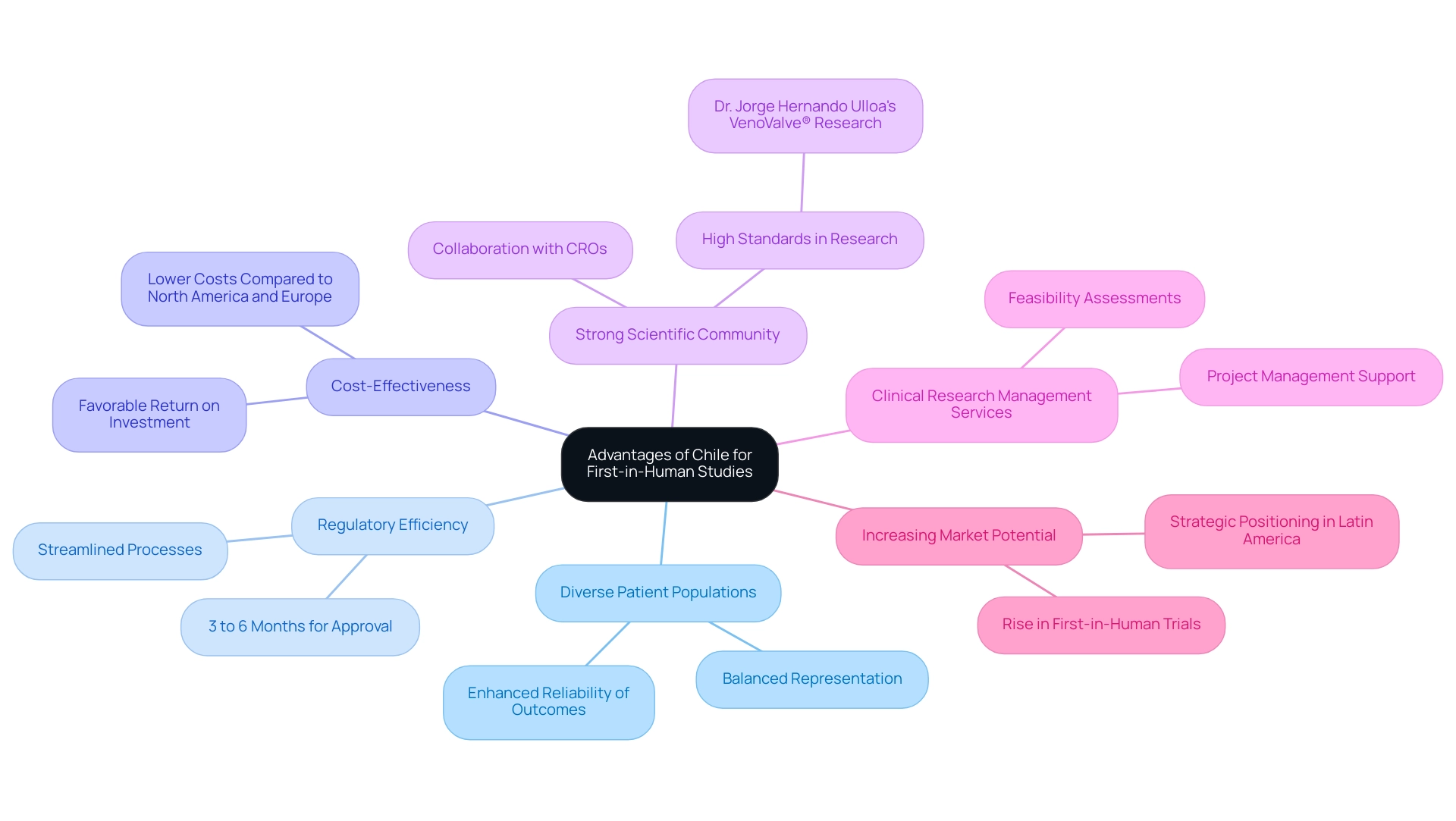
- Access to Diverse Patient Populations: The country's demographic diversity is an asset for recruiting participants who meet specific inclusion criteria, making it easier to conduct comprehensive and representative studies. In 2024, the demographics of research participants in Chile show a balanced representation across various age groups and health conditions, further enhancing the reliability of outcomes.
- Regulatory Efficiency: Chile's regulatory framework is notably streamlined, facilitating quicker approval processes than many other regions. The typical duration for regulatory consent of research studies in Chile is around 3 to 6 months, considerably less than in North America or Europe. This efficiency can greatly reduce the time needed to start medical studies, enabling sponsors to hasten their research schedules. The regulatory processes are designed to ensure compliance while promoting innovation, making Chile an attractive option for conducting early-feasibility and first-in-human studies.
- Cost-Effectiveness: Conducting clinical trials in Chile often proves to be more cost-effective than in North America or Europe. This financial advantage offers a favorable return on investment for sponsors, making it an economically attractive option.
- Strong Scientific Community: With a robust network of esteemed academic institutions and research centers, Chile fosters collaboration between Contract Research Organizations (CROs) and local experts. This synergy enhances the credibility and execution of investigations, ensuring high-quality research outcomes. Notably, Dr. Jorge Hernando Ulloa's presentation of one-year data from the VenoValve® research at the Charing Cross International Symposium exemplifies the high standards of vascular research in the region.
- Comprehensive Clinical Research Management Services: bioaccess® provides a range of services designed to assist research projects in Chile, including feasibility assessments, site selection, compliance evaluations, project setup, import permits, project management, and reporting. Their expertise ensures that stakeholders receive the necessary guidance and support throughout the trial process.
- Increasing Market Potential: As Latin America gains acknowledgment for its research capabilities, carrying out trials in Chile positions sponsors advantageously within this expanding market. Current trends suggest a rise in first-in-human trials, highlighting the nation's increasing significance in the global research environment, as demonstrated by ReGelTec's successful Early Feasibility Assessment on HYDRAFIL™ for addressing chronic low back pain. This strategic placement enhances their global strategy and opens doors to new opportunities.
These advantages, coupled with Chile's commitment to ethical research practices—such as ensuring the protection of vulnerable patient populations and upholding the principles of voluntary informed consent—make it an ideal choice for stakeholders considering first-in-human studies. The NeuroStim study exemplifies this potential, with 70% of participants reporting significant reductions in depression symptoms, underscoring the effectiveness of medical research conducted in the region. As Julio G. Martinez-Clark, CEO of Bioaccess, observes,
Chile has consistently achieved retention rates exceeding 85% in research trials, surpassing global averages.
By utilizing these unique opportunities and the extensive support provided by bioaccess®, stakeholders can make informed decisions about choosing a CRO that meets regulatory requirements while capitalizing on the advantages that Chile offers for first-in-human trials.
Evaluating Potential CROs: Key Criteria to Consider
When assessing potential clinical research organizations (CROs) for first-in-human studies in Latin America, it is essential to consider several key criteria that can significantly impact the success of the trial:
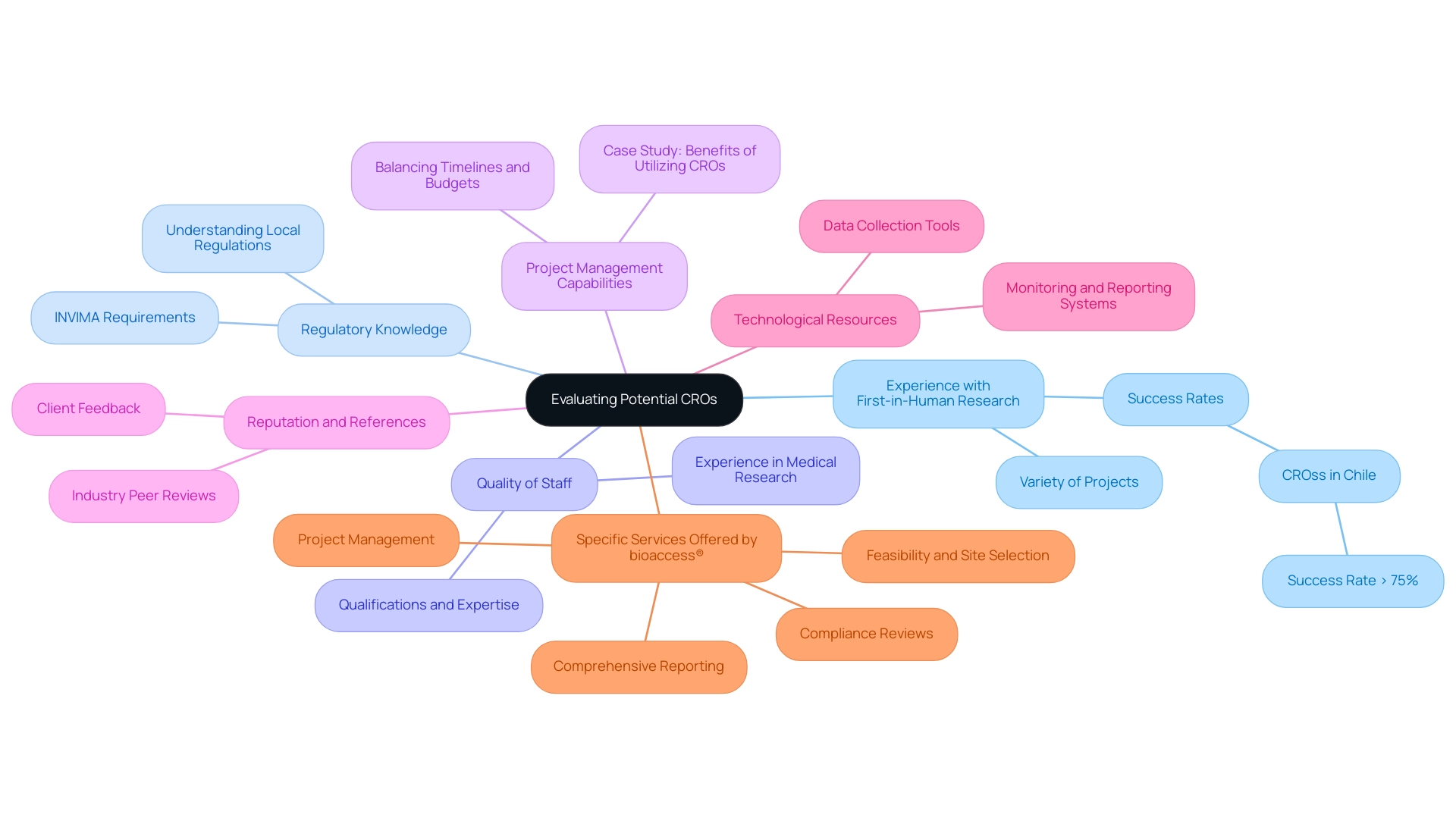
- Experience with First-in-Human Research: Assess the CRO's history in executing first-in-human experiments, including the variety of projects overseen and their related success rates. For example, recent assessments reveal that CROss in Chile have attained success rates exceeding 75% in first-in-human research, demonstrating their proficiency in managing intricate experiments. CROs with extensive experience in this area are often better equipped to manage the complexities involved.
- Regulatory Knowledge: A thorough understanding of local regulations is crucial. Ensure that the CRO is well-versed in the regulatory landscape of Latin America, particularly the requirements set by INVIMA (Colombia National Food and Drug Surveillance Institute), which oversees the marketing and manufacturing of health products. This knowledge can facilitate a smoother and more efficient approval process, significantly reducing the time to market for new therapies.
- Quality of Staff: Assess the qualifications and expertise of the CRO’s team. The depth of experience in medical research and management among personnel is a strong indicator of the organization’s ability to carry out studies effectively. Experienced teams can navigate challenges more adeptly, ensuring compliance and quality throughout the clinical research process.
- Project Management Capabilities: Identify CROss that exhibit robust project management skills. Their capacity to balance timelines, budgets, and resources is crucial for the prompt execution of studies, especially in the fast-paced realm of drug development. According to the case study titled "Benefits of Utilizing CROss," effective project management is a key factor in achieving successful outcomes and cost-effectiveness.
- Reputation and References: Gather feedback from prior clients and industry peers to evaluate the CRO's market reputation. A history of successfully meeting client expectations is a promising sign of reliability. As Laurie Stone, director at Clarkston Consulting, states, "Without CROss, the fast-paced nature of drug development would not be possible, so it is best to embrace the challenges and work with them to see success in delivering an effective, high-quality clinical study."
- Technological Resources: Consider the technological infrastructure that the CRO utilizes. Advanced tools and systems for data collection, monitoring, and reporting can significantly enhance efficiency and data integrity.
- Specific Services Offered by bioaccess®: bioaccess® provides essential services such as feasibility and selection of research sites and principal investigators, compliance reviews, study setup, import permits, project management, and comprehensive reporting. Their proficiency in managing these procedures is essential for successful medical studies.
By meticulously reviewing these criteria, stakeholders can make informed decisions when selecting a CRO that aligns with their research objectives, thereby increasing the likelihood of successful outcomes in first-in-human trials. Moreover, leveraging the expertise of bioaccess®, with over 20 years of experience in the Medtech field, can provide the necessary support in navigating the complexities of clinical trials and regulatory compliance in Latin America.
Building Strong Partnerships: Communication and Collaboration
To forge robust partnerships with Contract Research Organizations (CROs), implementing effective strategies is essential:
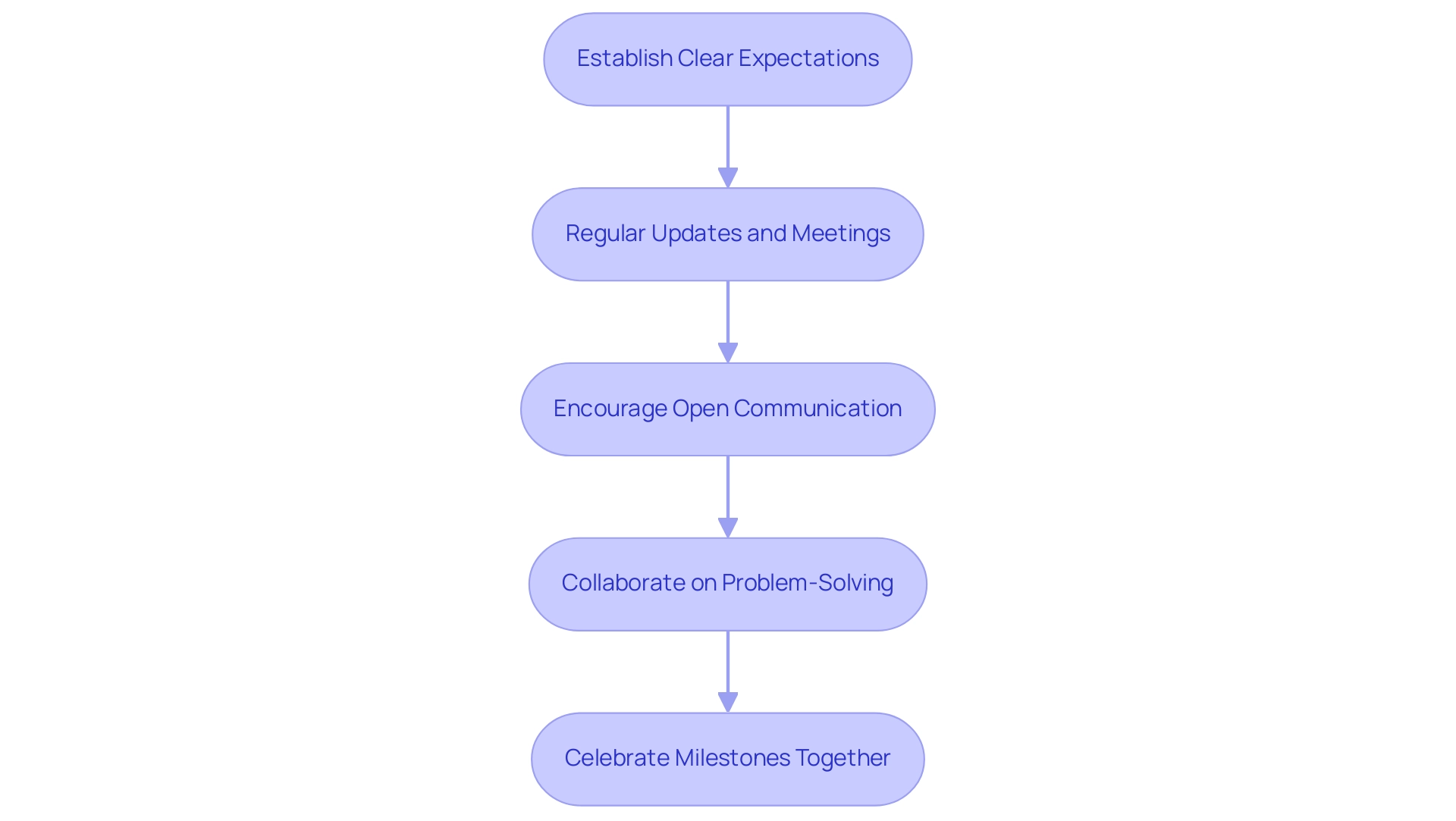
- Establish Clear Expectations: Clearly communicate your learning goals, timelines, and budget constraints from the outset. This alignment is crucial for setting a solid foundation for collaboration, especially in navigating complex regulatory environments for Early-Feasibility and First-In-Human studies.
- Regular Updates and Meetings: Organize consistent meetings to review progress, address any concerns, and make necessary adjustments. This practice keeps all stakeholders informed and engaged throughout the research, which is vital for compliance with local regulations, including those set by INVIMA.
- Encourage Open Communication: Create an atmosphere where team members feel empowered to share ideas, feedback, and concerns. Such open conversation can result in innovative solutions and enhance overall project execution, especially in the context of medical device evaluations where flexibility is essential.
- Collaborate on Problem-Solving: In the face of challenges, work hand-in-hand with the CRO to find solutions. This collaborative approach not only enhances trust but also fosters more effective problem resolution, a critical aspect when dealing with the complexities of clinical trial management, including site selection and compliance reviews.
- Celebrate Milestones Together: Acknowledge and celebrate key milestones throughout the research. Doing so not only boosts morale but also reinforces the partnership.
Examples of Successful Collaborations: For instance, a recent partnership between a biopharmaceutical sponsor and a CRO resulted in the successful completion of a first-in-human trial ahead of schedule. The teams held bi-weekly meetings to ensure alignment and transparency, which proved vital in navigating regulatory hurdles and adhering to INVIMA’s guidelines.
Case Study: In another case, a CRO implemented a real-time communication tool that allowed for instant updates and feedback, significantly improving the speed of decision-making and enhancing study outcomes. This case illustrates the importance of adapting communication strategies to fit the needs of the research and the stakeholders involved, especially in the regulatory landscape of medical devices.
By prioritizing clear communication and collaboration, stakeholders can significantly enhance their relationships with CROs. As highlighted by Linda Fischer, Executive Advisor at Backline by DrFirst, > The physician or clinician can communicate to anyone who needs to know, regardless of the borders. < This principle is especially relevant in the context of first-in-human research, where effective communication can significantly impact success rates. Moreover, a recent analysis revealed that 70% of healthcare providers found that asynchronous communication platforms enhanced interprofessional communication, emphasizing the vital role of effective messaging strategies in research.
Specific Services Offered by bioaccess®: Alongside the strategies noted, bioaccess® offers extensive management services for research projects, including setup, project oversight, and reporting. These services are crucial for ensuring adherence and successful implementation of medical research in the Latin American regulatory landscape.
Highlighting Proven Track Record: The expertise of bioaccess® is further illustrated through case studies showcasing successful collaborations that have navigated the complexities of regulatory environments and achieved timely project completions.
Assessing Post-Study Outcomes: Evaluating CRO Performance
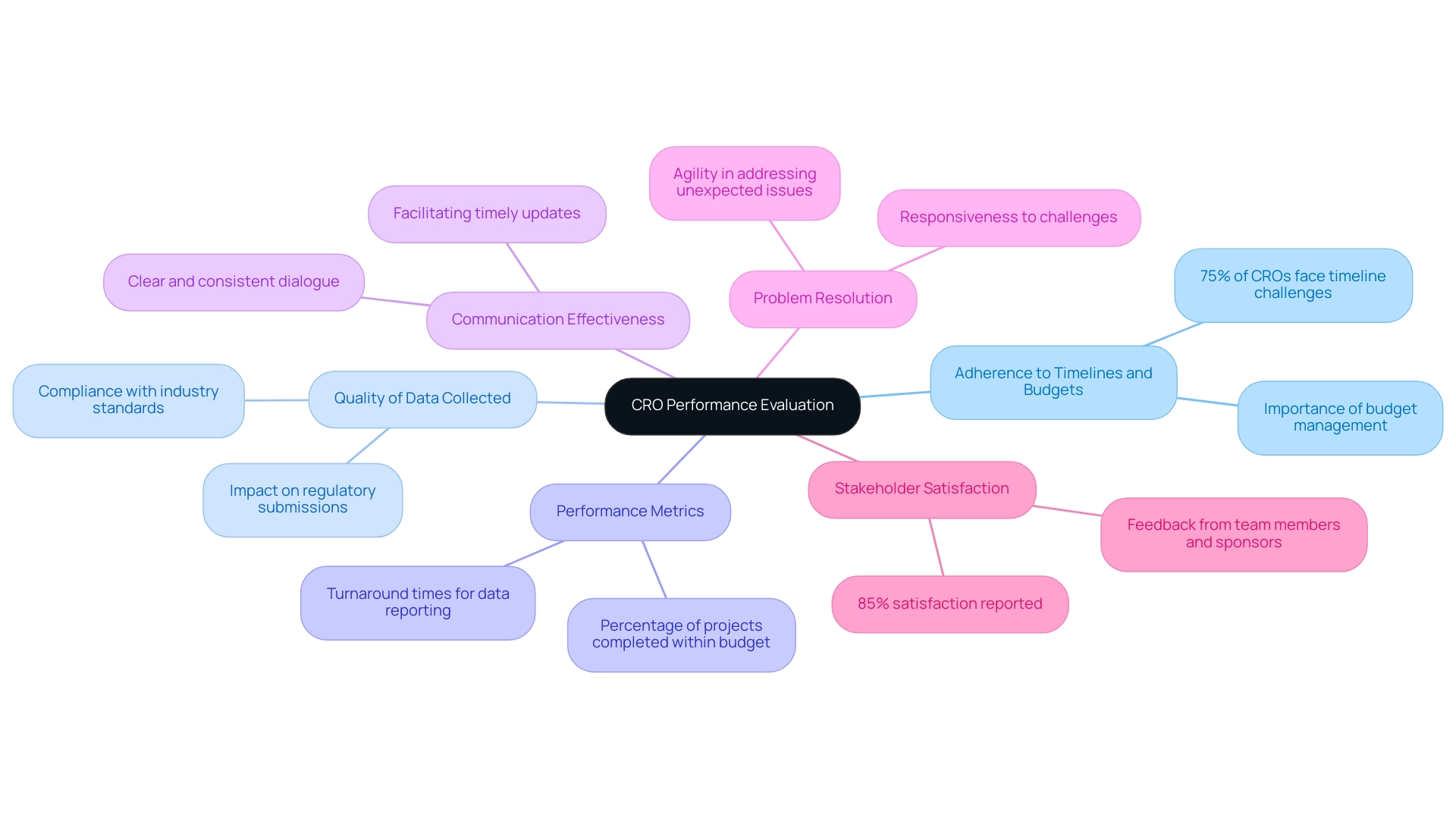
Following the completion of a first-in-human study, a comprehensive assessment of the Contract Research Organization (CRO), such as bioaccess™, is crucial to inform future collaborations. This evaluation should focus on the following criteria:
- Adherence to Timelines and Budgets: It is vital to examine whether the CRO adhered to the established timelines and budgetary constraints. Recent evaluations show that adherence to these metrics has become increasingly critical, with 75% of CROs reporting challenges in meeting them in 2024. Effective management of timelines and budgets is essential for determining the operational efficiency of CROs, thereby maintaining clinical study integrity.
- Quality of Data Collected: An evaluation of the data's quality and integrity is essential, as high-quality data underpins regulatory submissions and ensures scientific validity. The collection processes should be scrutinized to confirm compliance with industry standards and protocols, significantly impacting the results.
- Performance Metrics: In 2024, key performance metrics for CROs include turnaround times for data reporting and the percentage of projects completed within budget. Tracking these metrics provides insights into the CRO's efficiency and effectiveness in managing clinical trials, especially in complex environments like Latin America.
- Communication Effectiveness: Evaluating the effectiveness of communication throughout the research is paramount. Successful collaborations rely on clear and consistent dialogue between stakeholders and the CRO, facilitating timely updates and the resolution of potential issues.
- Problem Resolution: Reflecting on how the CRO managed challenges during the research offers insights into their responsiveness and problem-solving capabilities. An effective CRO, such as bioaccess™, should demonstrate agility in addressing unexpected issues, indicative of their operational effectiveness.
- Stakeholder Satisfaction: Gathering comprehensive feedback from all stakeholders—including team members, sponsors, and study participants—provides a holistic view of the CRO's performance. Recent surveys indicate that 85% of stakeholders reported satisfaction with CRO communication and problem resolution, highlighting areas for potential improvement in future projects.
By systematically evaluating these critical aspects, stakeholders can derive meaningful insights into the effectiveness of their CRO partnerships, particularly with specialized providers like bioaccess™, which boasts over 20 years of experience in Medtech. This extensive background enhances their credibility and underscores their flexibility in managing trials, thereby making informed decisions regarding future clinical trials.
Conclusion
Chile's position as a leading destination for clinical trials, particularly first-in-human studies, is underpinned by a robust regulatory framework and a commitment to ethical research practices. The country's streamlined approval processes, diverse patient populations, and cost-effective trial management create a unique environment that accelerates research timelines while ensuring compliance with international guidelines.
The pivotal role of Contract Research Organizations (CROs) cannot be overstated. By providing expertise in navigating regulatory landscapes, facilitating site selection, and ensuring adherence to ethical standards, CROs like bioaccess® empower sponsors to make informed decisions. Evaluating potential CROs based on their experience, regulatory knowledge, and project management capabilities is crucial for optimizing trial outcomes.
Moreover, fostering strong partnerships through effective communication and collaboration enhances the likelihood of success in clinical research endeavors. Regular updates, open dialogue, and joint problem-solving efforts create a cooperative atmosphere that is essential for navigating the complexities of clinical trials.
Ultimately, as the clinical research landscape in Latin America continues to evolve, Chile stands out as a prime location for sponsors seeking to leverage its unique advantages. By capitalizing on the expertise of local CROs and adhering to best practices in trial management, stakeholders can maximize their opportunities for success in this promising market.
Frequently Asked Questions
What regulations must research studies in Chile adhere to?
Research studies in Chile must adhere to regulations established by the Instituto de Salud Publica (ISP) and the ethics committees supervising research activities.
What is the regulatory approval process for clinical studies in Chile?
Before starting a clinical study, approval from the ISP must be obtained by submitting a detailed protocol that outlines the study's objectives, methodology, and safety measures.
Why is feasibility and site selection important in clinical studies?
Feasibility and site selection are crucial for the success of a study, as they determine the research locations and principal investigators (PIs) involved.
What role do ethics committees play in medical studies in Chile?
All medical studies must obtain ethical approval from an Institutional Review Board (IRB) to ensure the rights and welfare of participants are protected.
What is informed consent, and why is it important?
Informed consent is a process to ensure participants are fully aware of the study and agree to participate, which is a critical requirement in all research studies.
What are the obligations regarding adverse event reporting in clinical trials?
Researchers must familiarize themselves with the obligations for reporting adverse events and serious adverse events to regulatory authorities to maintain trial integrity and safety.
What requirements exist for import permits and nationalization of investigational devices in Chile?
Researchers must understand and comply with the requirements for obtaining import permits and nationalizing investigational devices to ensure operational success in research trials.
How does Chile's regulatory framework benefit clinical research?
Chile's regulatory framework is streamlined, facilitating quicker approval processes, which typically take around 3 to 6 months, enabling sponsors to expedite their research schedules.
What advantages does Chile offer for conducting first-in-human research?
Chile offers access to diverse patient populations, regulatory efficiency, cost-effectiveness, a strong scientific community, and comprehensive clinical research management services.
What is the significance of bioaccess® in clinical research in Chile?
Bioaccess® provides essential services such as feasibility assessments, site selection, compliance evaluations, project management, and reporting, supporting stakeholders throughout the trial process.
What should stakeholders consider when assessing potential CROs for first-in-human studies?
Stakeholders should consider the CRO's experience with first-in-human research, regulatory knowledge, quality of staff, project management capabilities, reputation, technological resources, and specific services offered.
How can effective collaboration with CROs enhance clinical research outcomes?
Establishing clear expectations, maintaining regular updates, encouraging open communication, collaborating on problem-solving, and celebrating milestones together can significantly improve partnerships with CROs.

