Introduction
As the landscape of clinical trials continues to evolve, Paraguay is emerging as a notable player in the realm of first-in-human studies. With a regulatory framework that is becoming increasingly conducive to clinical research, the country offers a wealth of opportunities for Medtech companies looking to expand their trials into Latin America. However, navigating this promising terrain is not without its challenges, including regulatory complexities and resource fragmentation.
This article delves into the factors that are driving the growth of clinical trials in Paraguay, the essential considerations for selecting a Contract Research Organization (CRO), and the importance of fostering strong relationships to ensure successful outcomes in this burgeoning market. Through a comprehensive exploration of these themes, stakeholders can gain valuable insights into effectively managing clinical research initiatives in Paraguay.
The Emerging Landscape of Clinical Trials in Paraguay
Paraguay is rapidly establishing itself as a prime destination for clinical trials, especially in the context of First-in-Human Studies CRO Paraguay. The nation's oversight framework is undergoing significant evolution, fostering an environment increasingly supportive of clinical research initiatives. However, US Medtech companies face challenges such as regulatory hurdles, language barriers, and the fragmentation of resources, which can complicate their efforts in Latin America.
Key factors driving the trend in Paraguay include:
- A growing population of treatment-naive patients
- Enhanced healthcare infrastructure
- A pool of qualified healthcare professionals
The collaboration between Greenlight Guru and bioaccess™ enhances this landscape by streamlining processes and reducing risks for Medtech manufacturers, while also providing essential services such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Project management
Significantly, the cost-effectiveness of carrying out assessments in Paraguay, when contrasted with North America and Europe, offers an appealing choice for sponsors.
As highlighted by Researcher B, the time to clinical study approval is between 6 to 8 weeks, underscoring the efficiency of the current system. Furthermore, a recent case analysis titled 'Recommendations for Improving Clinical Research in Paraguay' emphasizes the need for enhancing the regulatory framework and increasing training for ethics review board members. This highlights the ongoing challenges and recommendations necessary for enhancing clinical research.
For stakeholders aiming to collaborate with the First-in-Human Studies CRO Paraguay, understanding these dynamics is crucial for navigating the complexities of this burgeoning market. Moreover, coordinated efforts are essential to further enhance health research, particularly emphasizing the need for a centralized policy to promote research and development (R&D) in the region. Importantly, authors of the research have declared no conflicts of interest related to the research conducted, ensuring transparency and trustworthiness in the context of clinical trials.
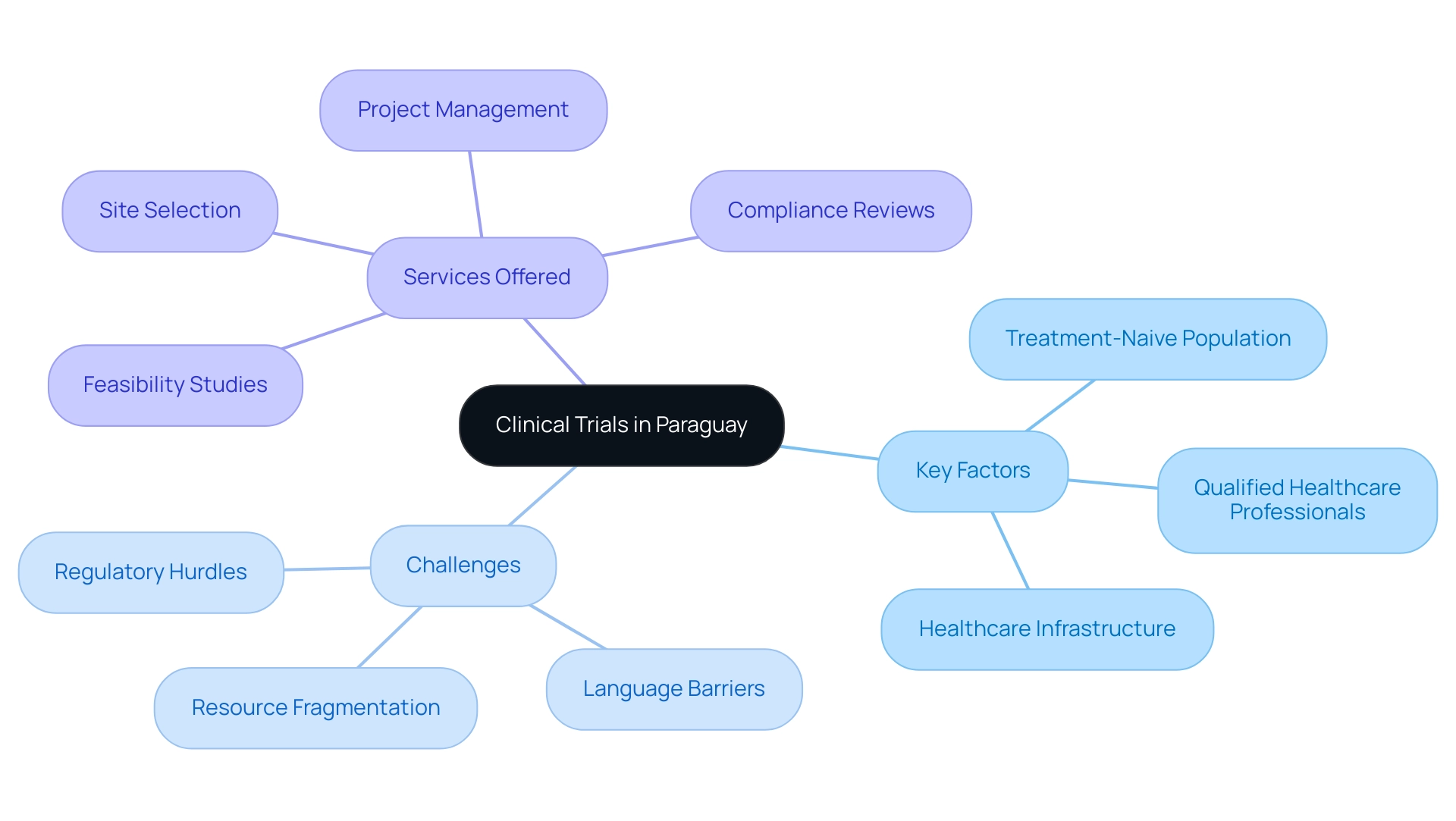
Key Considerations for Selecting a CRO for First-in-Human Studies
When choosing a CRO for first-in-human trials, especially in Colombia, consider the following key factors:
- Regulatory Expertise: Ensure the CRO has a deep understanding of local regulations and can efficiently navigate the approval processes. In Colombia, this includes a streamlined review process where the total IRB/EC and Ministry of Health (INVIMA) review takes just 90-120 days, minimizing delays and ensuring compliance with national and international standards.
- Experience with First-in-Human Studies: Look for a CRO with a proven track record in conducting first-in-human studies. Their extensive experience will be invaluable in managing the unique challenges associated with these trials.
- Local Knowledge and Network: A CRO with strong connections within the local healthcare community can facilitate patient recruitment, essential for studies in Colombia, which boasts over 50 million residents and universal healthcare coverage for 95% of the population. This local insight can significantly enhance the quality of data gathering and success of the study.
- Quality Assurance Processes: Assess the CRO’s quality management systems to ensure adherence to Good Clinical Practice (GCP) and robust monitoring and reporting mechanisms. Colombia's healthcare system is recognized for its quality, ranked #22 by the WHO among 191 countries, further ensuring high standards.
- Experiment Setup and Approval Procedures: Assess the CRO’s abilities in experiment preparation, including the review and feedback on research documents to meet country requirements, and their management of import permits and nationalization of investigational devices.
- Flexibility and Responsiveness: Select a First-in-Human Studies CRO that demonstrates agility in adapting to project needs and changes, as first-in-human studies often require quick adjustments based on evolving circumstances.
By thoroughly evaluating these considerations, stakeholders can choose a First-in-Human Studies CRO that aligns with their goals and ensures the successful execution of first-in-human studies in Colombia. For more information on our comprehensive clinical trial management services, BOOK A MEETING with us today.
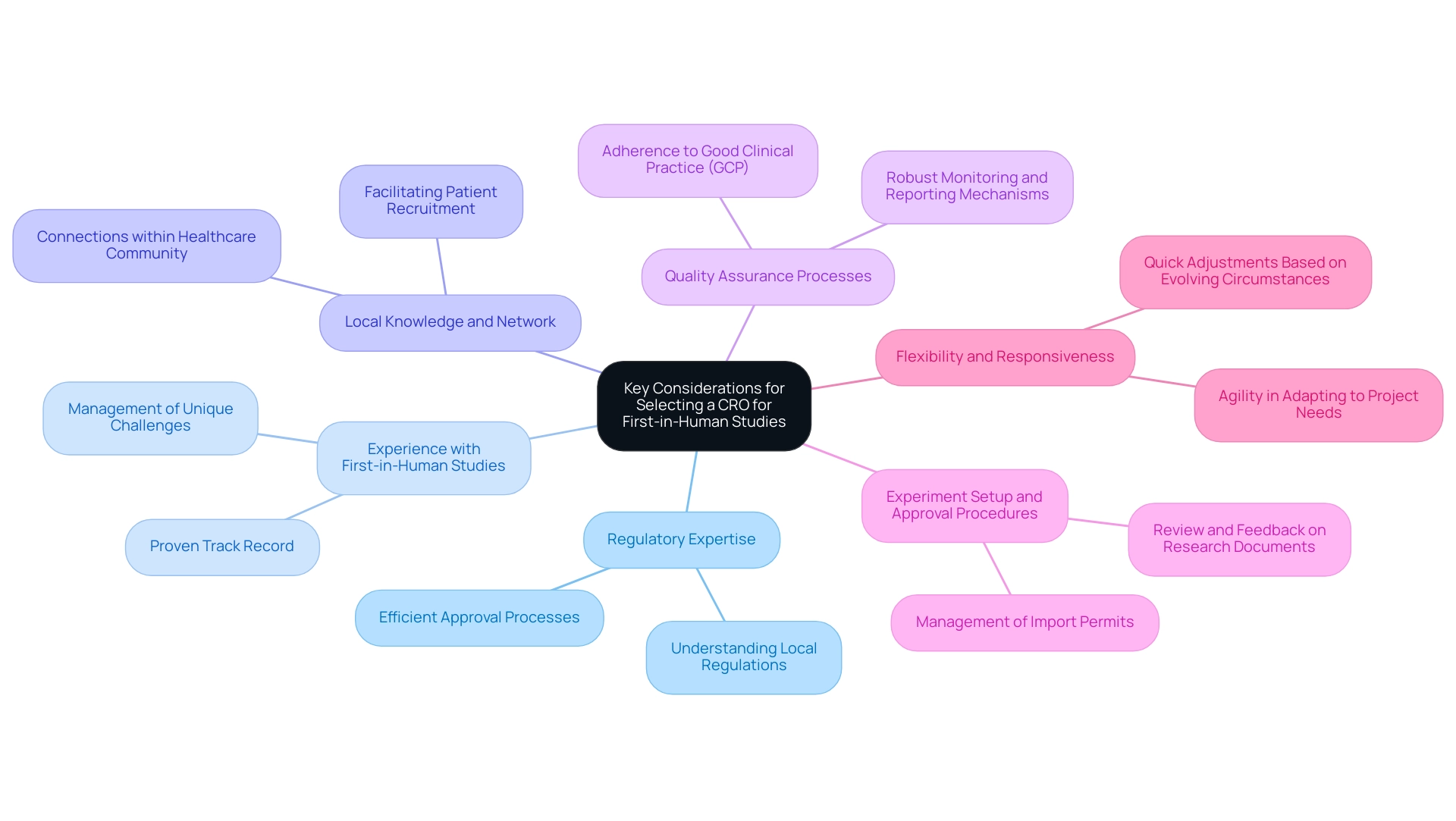
Evaluating CRO Capabilities and Resources
When evaluating the First-in-Human Studies CRO Paraguay for navigating regulatory environments for First-in-Human and Early-Feasibility Studies in Latin America, consider the following aspects of their capabilities and resources:
- Staff Expertise: Review the qualifications and experience of the CRO’s team members, particularly those who will be directly involved in the study. With more than 20 years in Medtech, bioaccess® has a team skilled in pertinent therapeutic fields and clinical study management.
- Technological Infrastructure: Assess the CRO's use of technology for data management, patient monitoring, and communication. A CRO that utilizes advanced systems can improve efficiency and data accuracy, essential for successful study outcomes.
- Patient Recruitment Strategies: Inquire about the CRO's approaches for patient recruitment and retention, as these are vital for the success of first-in-human trials. A robust recruitment plan can significantly reduce timelines and enhance research results, showcasing the CRO's commitment to effective trial management.
- Site Management Capabilities: Evaluate the CRO’s experience in managing clinical sites, including their ability to train site staff and ensure compliance with study protocols. bioaccess® excels in feasibility and selection of research sites, ensuring alignment with regulatory requirements.
- Service Capabilities: Consider the CRO's comprehensive service offerings, including study setup, compliance reviews, import permits, and project management. These elements are crucial for the smooth execution of clinical trials.
- Financial Stability: Consider the financial health of the CRO, as this can impact their ability to deliver on commitments and manage project budgets effectively. A stable CRO, such as bioaccess®, can provide the assurance needed in navigating the complexities of clinical trials.
- Vetted Status: Acknowledge that bioaccess™ is a vetted CRO and consulting partner for U.S. medical device companies, which enhances their credibility and capability in managing trials in Latin America.
By thoroughly evaluating these factors, stakeholders can ensure that the First-in-Human Studies CRO Paraguay they select, like bioaccess®, is well-equipped to handle the complexities of first-in-human trials and accelerate the clinical development process.
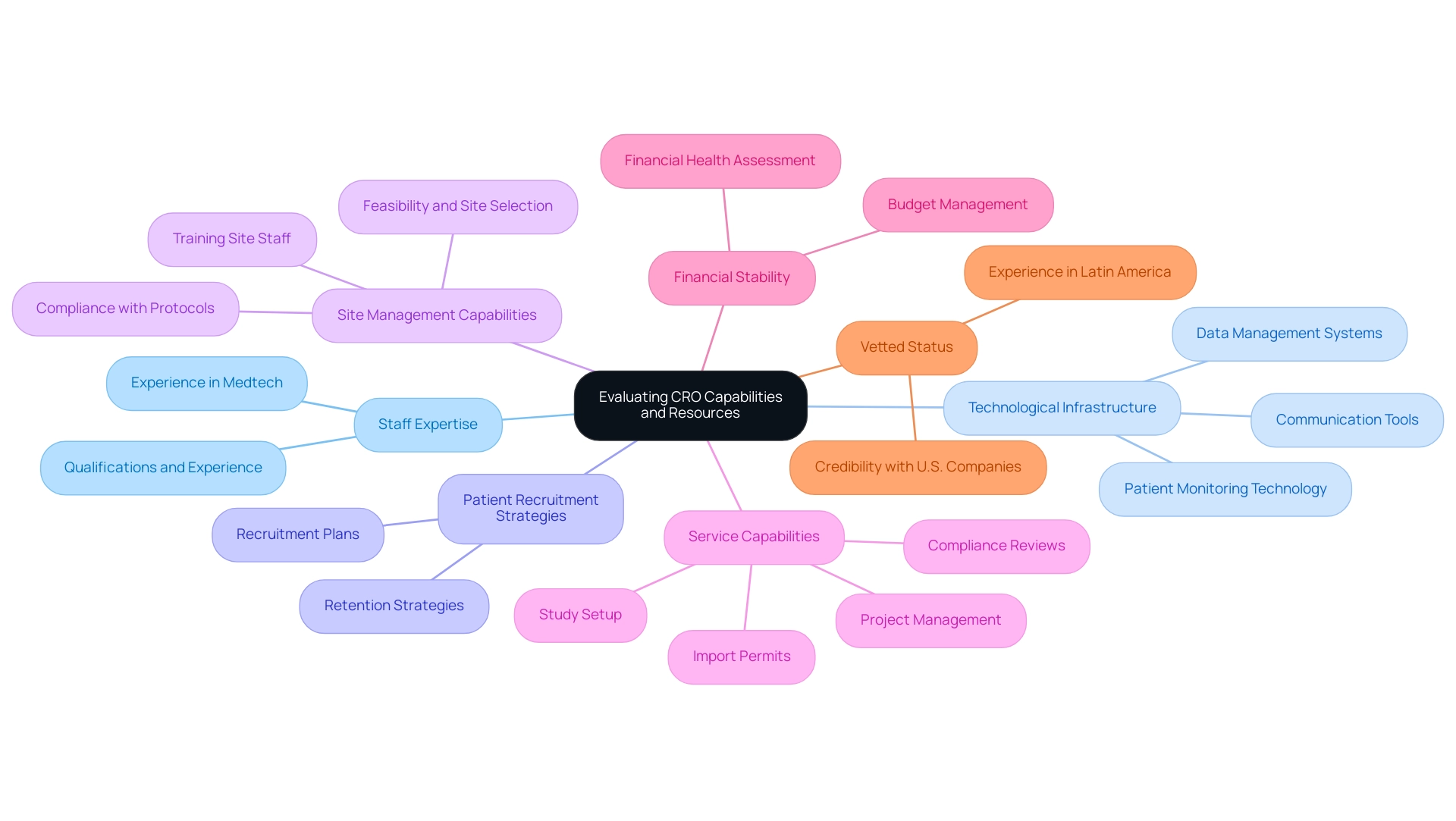
Understanding Regulatory Compliance and Ethical Considerations
It is essential to ensure that the CRO you select has a robust understanding of compliance and ethical considerations:
- Informed Consent Processes: Verify that the CRO has established procedures for obtaining informed consent from participants, ensuring they fully understand the risks and benefits of the research.
- Adherence to GCP: Confirm that the CRO follows Good Clinical Practice (GCP) guidelines, which are critical for ensuring the safety and well-being of participants and the integrity of data.
- Regulatory Submissions: Verify the experience of First-in-Human Studies CRO Paraguay in preparing and submitting compliance documents to local authorities, as this is a vital step in starting research. With our extensive service capabilities, we ensure compliance with country requirements and provide thorough feedback on research documents, including assistance with obtaining import permits and nationalization of investigational devices.
- Ethics Committee Engagement: Evaluate the capability of the First-in-Human Studies CRO Paraguay to interact with local ethics committees and oversight bodies, ensuring that all necessary approvals are secured before initiating the research. Our experienced team, including Ana Criado, a compliance affairs expert and former leader at INVIMA, is adept at navigating these processes.
- Monitoring and Reporting: Ensure that the CRO has robust monitoring and reporting systems in place to track compliance throughout the research, addressing any issues that may arise promptly. Our extensive project management services encompass thorough reporting on research status, inventory, and adverse events.
By concentrating on these compliance and ethical factors, stakeholders can guarantee that their chosen First-in-Human Studies CRO Paraguay operates with the utmost standards of integrity and adherence, supported by the expertise of professionals like Ana Criado and Katherine Ruiz, who possess significant experience in compliance matters for medical devices and clinical trials in Colombia.
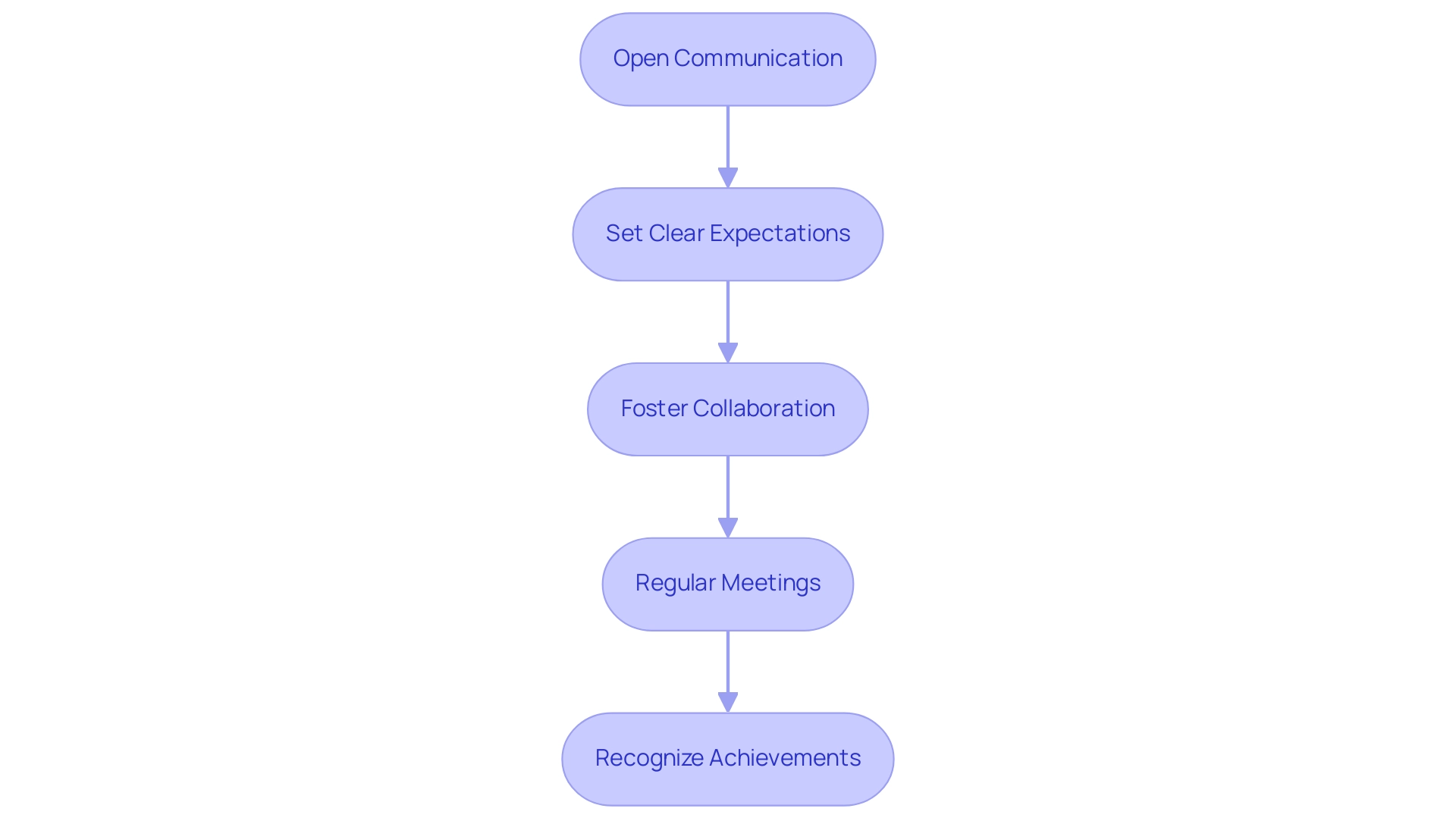
Building Strong Relationships with CROs
To establish a solid connection with your chosen CRO, particularly while managing the intricate compliance landscapes for first-in-human and early-feasibility research in Latin America, contemplate the following strategies:
- Open Communication: Establish clear lines of communication from the outset. Frequent updates and transparent communication can assist in resolving problems before they worsen, especially considering the complex nature of compliance requirements for medical device trials. bioaccess® highlights the significance of communication throughout the design and submission processes to ensure compliance with local regulations.
- Set Clear Expectations: Clearly define roles, responsibilities, and expectations for both parties. This clarity will help prevent misunderstandings and ensure alignment throughout the research, especially when dealing with diverse regulatory submissions to ethics committees and health ministries. bioaccess® provides detailed guidelines and support for these submissions to streamline the process.
- Foster Collaboration: Encourage a collaborative environment where both teams can share insights and feedback. This partnership approach can lead to innovative solutions, particularly in overcoming challenges such as recruitment issues and competition from established players in the field. bioaccess® offers project management services that facilitate this collaboration by keeping all stakeholders informed and engaged.
- Regular Meetings: Schedule regular meetings to discuss progress, challenges, and next steps. These check-ins can help maintain momentum and keep everyone aligned, which is crucial for successful trial management and monitoring, including reporting on both serious and non-serious adverse events. bioaccess® ensures that these meetings are part of their comprehensive monitoring and reporting services.
- Recognize Achievements: Acknowledge and celebrate milestones and successes throughout the course. Recognizing the contributions of the CRO can foster goodwill and strengthen the partnership, ultimately benefiting the broader local economy through job creation and improved healthcare outcomes.
By implementing these strategies and leveraging the specific services offered by bioaccess®, stakeholders can cultivate strong relationships with CROs that enhance collaboration and lead to successful First-in-Human Studies CRO Paraguay.
Conclusion
Paraguay is positioning itself as an increasingly attractive destination for clinical trials, particularly first-in-human studies, as evidenced by its evolving regulatory framework and growing healthcare infrastructure. The opportunities presented by the country are compelling, particularly for Medtech companies aiming to expand their footprint in Latin America. Key factors such as a treatment-naive patient population, qualified healthcare professionals, and the collaboration with experienced Contract Research Organizations (CROs) like bioaccess™ are pivotal in facilitating efficient trial management and enhancing the potential for successful outcomes.
However, stakeholders must remain cognizant of the challenges that accompany this promising landscape. Navigating regulatory complexities, addressing language barriers, and overcoming resource fragmentation are critical considerations that can significantly impact the success of clinical trials in Paraguay. Selecting a CRO with robust local knowledge, regulatory expertise, and a proven track record is essential for ensuring compliance and optimizing trial execution.
Building strong relationships with CROs is equally vital. Open communication, clear expectations, and collaborative efforts can foster an environment conducive to innovation and problem-solving. By recognizing achievements and maintaining regular dialogue, stakeholders can reinforce partnerships that ultimately contribute to the broader goals of advancing healthcare research and improving patient outcomes.
In conclusion, Paraguay's emergence as a hub for clinical trials offers significant opportunities for Medtech companies, provided that they navigate the landscape with strategic foresight and a commitment to collaboration. By leveraging local expertise and fostering strong partnerships, stakeholders can effectively manage the complexities of clinical research initiatives, paving the way for successful trials that benefit both the local population and the global medical community.
Frequently Asked Questions
Why is Paraguay becoming a popular destination for clinical trials?
Paraguay is rapidly establishing itself as a prime destination for clinical trials due to its evolving oversight framework, a growing population of treatment-naive patients, enhanced healthcare infrastructure, and a pool of qualified healthcare professionals.
What challenges do US Medtech companies face when conducting clinical trials in Paraguay?
US Medtech companies encounter challenges such as regulatory hurdles, language barriers, and the fragmentation of resources, complicating their efforts in Latin America.
What services does the collaboration between Greenlight Guru and bioaccess™ provide for Medtech manufacturers?
The collaboration offers essential services including feasibility studies, site selection, compliance reviews, and project management, which streamline processes and reduce risks for Medtech manufacturers.
How does the cost of conducting clinical trials in Paraguay compare to North America and Europe?
Conducting assessments in Paraguay is more cost-effective compared to North America and Europe, making it an appealing choice for sponsors.
What is the average time for clinical study approval in Paraguay?
The time to clinical study approval in Paraguay is between 6 to 8 weeks, highlighting the efficiency of the current system.
What recommendations have been made to improve clinical research in Paraguay?
Recommendations include enhancing the regulatory framework and increasing training for ethics review board members to address ongoing challenges in clinical research.
What factors should stakeholders consider when collaborating with First-in-Human Studies CRO Paraguay?
Stakeholders should understand the regulatory environment, the capabilities of the CRO, and the need for coordinated efforts to enhance health research and promote research and development in the region.
What are key considerations when selecting a CRO for first-in-human trials in Colombia?
Key considerations include regulatory expertise, experience with first-in-human studies, local knowledge and network, quality assurance processes, flexibility, and responsiveness.
What are the essential compliance and ethical considerations when working with a CRO?
Essential considerations include informed consent processes, adherence to Good Clinical Practice (GCP), regulatory submissions, ethics committee engagement, and robust monitoring and reporting systems.
How can stakeholders establish a strong connection with their chosen CRO?
Stakeholders can establish a strong connection through open communication, setting clear expectations, fostering collaboration, scheduling regular meetings, and recognizing achievements throughout the research process.

