Overview
Choosing the right Clinical Research Organization (CRO) for your clinical trials involves assessing factors such as expertise, regulatory compliance, service offerings, and communication skills. The article outlines a step-by-step approach that includes defining your needs, evaluating proposals, and maintaining open communication to ensure a successful partnership, emphasizing that thorough planning and informed decision-making are critical for navigating the complexities of clinical research.
Introduction
Selecting a Clinical Research Organization (CRO) is a pivotal step in the success of clinical trials, particularly when navigating the complexities of first-in-human medical devices in Colombia. With a myriad of options available, the decision-making process can be daunting. Key considerations such as:
- expertise
- regulatory compliance
- technological capabilities
play a crucial role in identifying the right partner. This article delves into essential factors and a systematic approach for selecting a CRO, assessing performance metrics, and fostering a strong collaborative relationship, ultimately ensuring that clinical trials are both efficient and compliant with industry standards. By understanding these elements, organizations can enhance their chances of achieving successful outcomes in their research endeavors.
Key Considerations for Selecting a Clinical Research Organization (CRO)
When choosing a Clinical Research Organization (CRO) for your study, particularly for first-in-human medical devices in Colombia, several key factors should be prioritized to ensure success:
- Expertise and Experience: Assess the CRO's history and effectiveness in managing clinical trials relevant to your therapeutic area. Organizations with demonstrated success in similar projects are more likely to meet your research's unique demands.
- Regulatory Compliance: It is imperative that the CRO adheres to all relevant regulations, including Good Clinical Practice (GCP) guidelines. Compliance is crucial for preserving the integrity of your research and safeguarding patient welfare, particularly in navigating the regulatory landscape governed by INVIMA, Colombia's national health authority that oversees medical device classification and trials.
- Services Offered: Evaluate the breadth of services that the CRO provides. A full-service CRO can streamline processes by offering comprehensive support from research design—including protocol writing and investigator's brochures—to data analysis, thus minimizing the need for multiple vendors. Services such as feasibility studies, site selection, setup for testing, import permits, project management, and monitoring should be included in their offerings. Additionally, ensure that the CRO can coordinate the clinical study agreement (CTA) and manage the submission of the import permit application, as these are critical for operational success.
- Technology and Infrastructure: Investigate the technological capabilities of the CRO, particularly their data management systems and electronic data capture (EDC) tools. The integration of advanced technology can significantly enhance efficiency and accuracy in data collection and reporting. As emphasized by industry specialists, employing tools that minimize site visits, such as electronic patient-reported outcomes (ePRO) and home visits, can also enhance enrollment for hard-to-reach patient populations. Notably, 58.5% of Hispanic and non-white individuals found home visits appealing, compared to only 38.4% of non-Hispanic and white participants, emphasizing the importance of diverse recruitment strategies.
- Team Qualifications: Scrutinize the qualifications of the CRO's personnel. It is crucial to ensure that team members possess the necessary expertise to manage your research effectively.
- Communication and Collaboration: Effective communication is essential for fostering a successful partnership. Assess the CRO’s engagement approach and responsiveness to your inquiries and needs.
- Cost and Budgeting: Finally, gain a clear understanding of the CRO's pricing structure. While cost should not be your only consideration, it is important to ensure that the services provided fit within your budget while maintaining high standards of quality. Additionally, given the challenges faced by medical device startups—such as regulatory hurdles, competition, recruitment issues, and financial constraints—it's vital to understand how to choose the right CRO for your clinical trials to effectively navigate these complexities. A 2019 Pew research survey found that 80% of internet users search for health information online. This statistic emphasizes the potential for targeted online recruitment strategies, especially as younger individuals are more likely to find studies through social media. Despite social media not being the primary source for study information, effective online targeting can engage patients interested in health topics, as illustrated in case studies on Clinical Study Recruitment through Social Media. A nuanced approach to communication is essential for successful recruitment, considering patient preferences across different demographics.
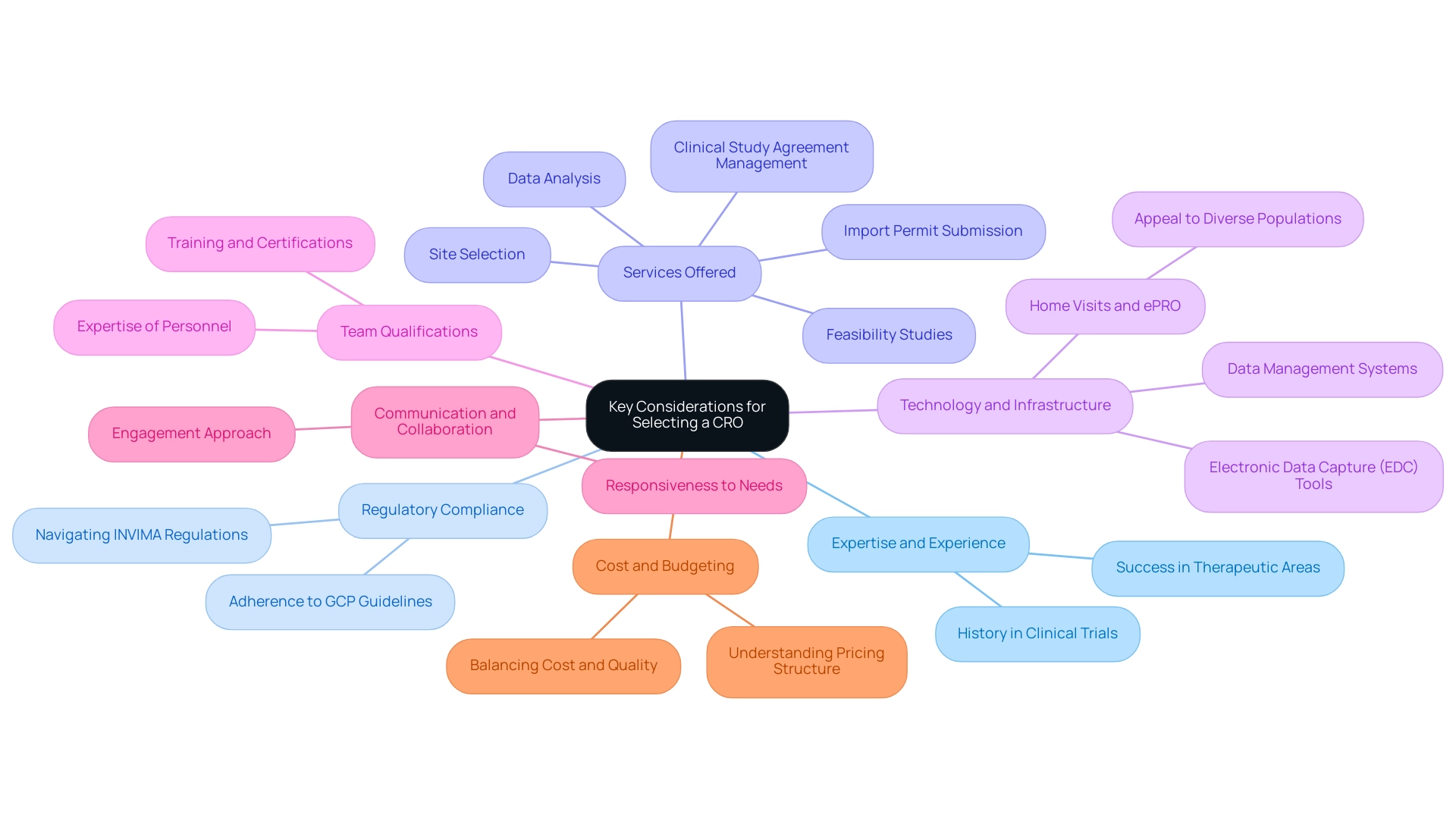
A Step-by-Step Approach to Choosing the Right CRO
It is essential for guaranteeing success to understand how to choose the right CRO for your clinical trials. Follow these steps to make an informed decision:
- Define Your Needs: Begin by articulating the objectives and requirements of your research study. This encompasses taking into account the therapeutic area, phases of research, and specific services the CRO must offer.
- Conduct Preliminary Research: Develop a list of potential CROs through recommendations, online research, and industry references. Prioritize organizations with a strong reputation, such as bioaccess®, which focuses on medical device studies in Latin America and has over 20 years of experience in Medtech.
- Request Proposals: Approach the shortlisted CROs to request comprehensive proposals. These should detail their services—including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF)—experience, team qualifications, and pricing structures to facilitate informed comparisons.
- Evaluate Proposals: Assess the proposals against key factors such as expertise in regulatory compliance and cost. Implement a scoring system to objectively analyze each CRO's strengths and weaknesses, ensuring a thorough evaluation. Confirm if the CRO performs internal audits and includes quality assurance personnel on study teams to maintain high standards.
- Schedule Interviews: Arrange interviews with the top candidates to discuss your project in detail. Evaluate their communication skills, responsiveness, and collaborative approach during these discussions.
- Check References: Solicit references from previous clients and follow up for insights into their experiences with the CRO. Inquire about performance, reliability, and adherence to deadlines to gauge the CRO’s track record.
- Make a Decision: After a comprehensive evaluation of all factors, choose the CRO that most effectively aligns with your project requirements and organizational objectives. It’s crucial that the chosen CRO has the required expertise, adaptability, and support abilities to guarantee the success of your research study, which is essential when considering how to choose the right CRO for your clinical trials.
- Negotiate Contract Terms: Upon selecting a CRO, engage in negotiations regarding contract terms to ensure clarity on deliverables, timelines, and payment structures. This critical step lays the foundation for a productive working relationship.
Patricio Ledesma, Head of Clinical Operations at Sofpromed CRO, emphasizes the significance of meticulous planning and execution in research studies, noting his dedication to assisting biotech leaders in navigating these intricate processes. It’s also vital to be aware that changing a CRO mid-study can introduce significant technical and financial risks, underscoring the importance of making the right choice from the outset. Furthermore, understanding specific services such as fill-finish processes, as outlined in the "Guide to Fill-Finish Services for Early Phase Clinical Trials in 2025," can greatly inform your selection.
Furthermore, recent discussions at the 35th International Symposium on ALS/MND, sponsored by Atlantic Research Group, emphasize how to choose the right CRO for your clinical trials in today’s research landscape.
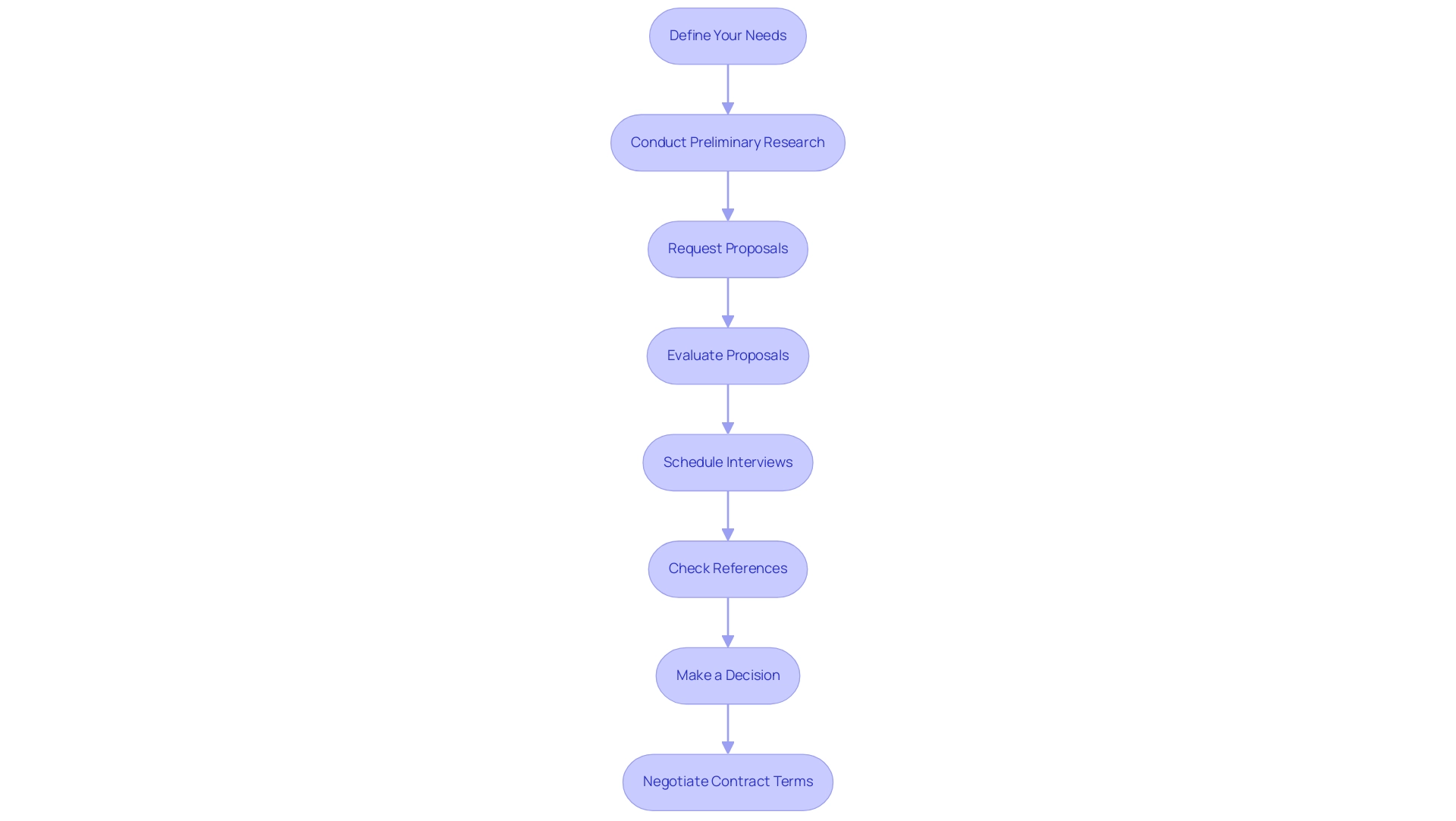
Assessing CRO Performance Metrics
To effectively evaluate the performance of a Clinical Research Organization (CRO), it is crucial to consider the following key metrics:
- Timeliness: Assess the CRO's capability to adhere to project timelines and deadlines. Delays can profoundly affect the overall success of a clinical study, underscoring the need for punctuality. For example, the case analysis titled "Time from Grant Award to First Accrual" emphasizes the significance of monitoring the duration from the grant award notice to the enrollment of the first subject, reflecting the efficiency of the research process.
- Quality of Data: Examine the accuracy and completeness of the data collected by the CRO. High-quality data is essential for generating trustworthy research results, directly impacting the validity of the outcomes.
- Regulatory Compliance: Scrutinize the CRO's adherence to regulatory requirements throughout the project lifecycle. Non-compliance can lead to severe repercussions, including trial delays or invalidation of results. This includes a thorough review and feedback on academic documents to ensure compliance with country requirements.
- Subject Recruitment and Retention: Evaluate the CRO's proficiency in recruiting and retaining study participants. A robust recruitment strategy is essential for study completion. Industry experts highlight that subject accrual is a significant challenge across the sector, as stated by Wendy Tate, PhD, GStat Product Strategy Director. Furthermore, utilizing Google Ads can be an effective method for enhancing recruitment efforts in clinical studies.
- Budget Adherence: Monitor the CRO's ability to remain within the agreed budget. Cost overruns present risks to the financial sustainability of the study and can jeopardize the overall research objectives.
- Client Satisfaction: Collect feedback from stakeholders involved in the test to gauge their satisfaction with the CRO's performance and communication. Positive relationships foster collaboration and enhance overall project success.
- Post-Study Support: Evaluate the level of assistance offered by the CRO after the study concludes, which includes data analysis and reporting. Effective post-trial support is essential for translating findings from bench to bedside, aligning with recent efforts by programs such as the CTSA to improve clinical research efficiency.
- Study Setup and Project Management: Consider the CRO's capabilities in study setup and project management. Efficient experiment setup guarantees that all essential preparations are completed for the research, while robust project management maintains the initiative on course and within limits.
By regularly tracking these performance metrics, organizations can ensure that the CRO remains aligned with project objectives, which is essential when considering how to choose the right CRO for your clinical trials, allowing them to swiftly address any issues that may occur and ultimately improving the quality and efficiency of research. Furthermore, leveraging comprehensive services like feasibility assessments, site selection, trial setup, project management, and reporting can significantly bolster trial integrity and outcomes, contributing positively to local economies through job creation and healthcare improvements.
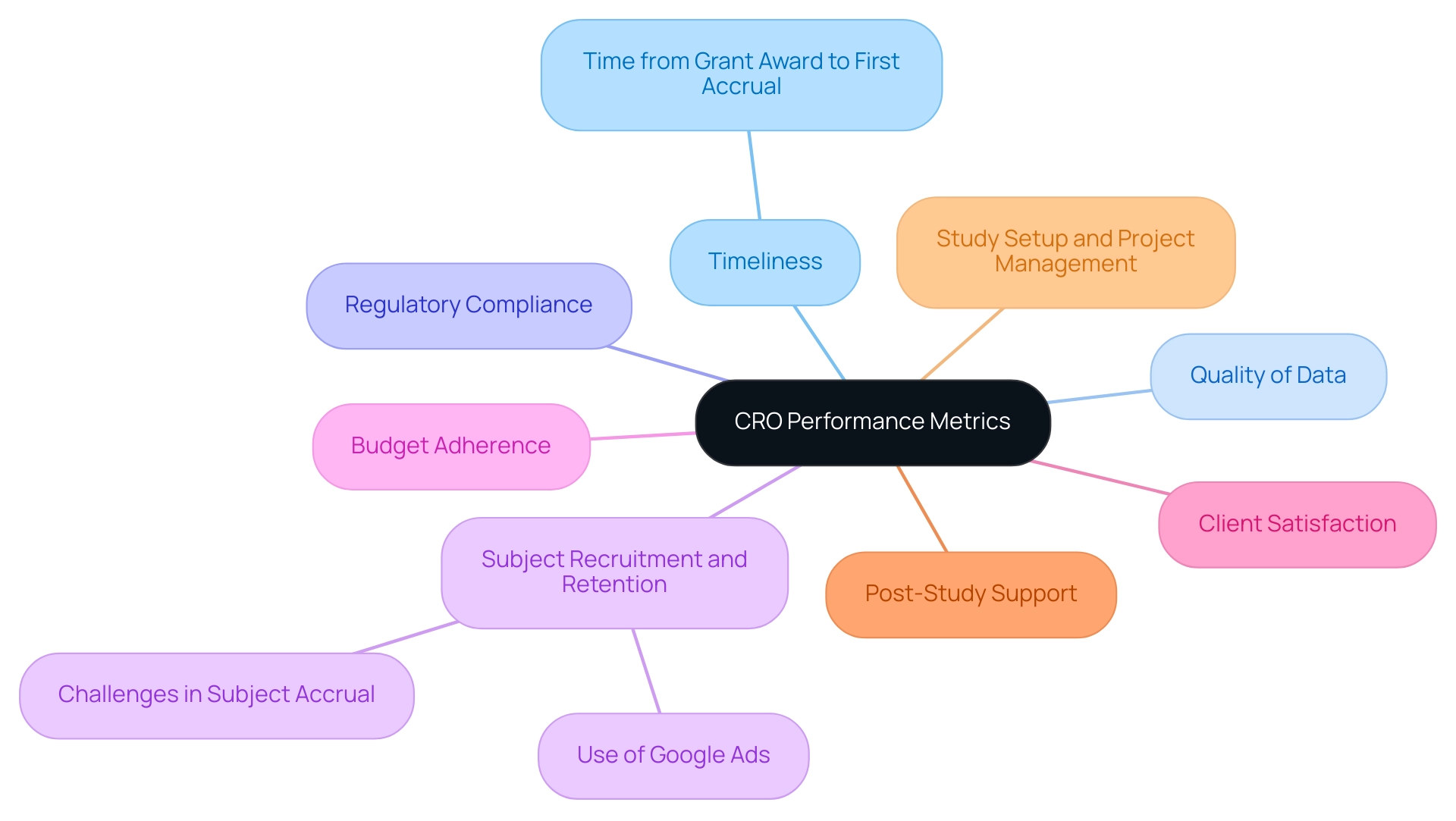
Building a Strong Relationship with Your CRO
To cultivate a robust partnership with your selected CRO, adhere to the following best practices:
- Set Clear Expectations: Initiate the partnership by articulating your goals, timelines, and expectations comprehensively. Establishing clarity from the outset is crucial in preventing potential misunderstandings down the line, especially regarding the feasibility and selection of research sites and principal investigators (PIs).
- Maintain Open Communication: Implement regular communication channels for discussing project updates, challenges, and feedback. Consistent check-ins not only keep all parties aligned but also foster a culture of transparency, which is essential in clinical research settings. Significantly, research discovered that 89% of individuals believe efficient communication is essential, yet 80% rate workplace communication as average or poor. This statistic underscores the importance of effective communication in CRO partnerships.
- Encourage Collaboration: Create an atmosphere that promotes collaboration by involving the CRO in decision-making processes. Their expertise in trial setup, compliance reviews, and project management can enhance engagement and lead to innovative solutions, as evidenced by companies like Google and HubSpot, which successfully leverage multiple communication channels to streamline internal operations.
- Provide Constructive Feedback: Regularly assess and provide feedback on the CRO's performance, including their review and comments on research documents. Engaging in positive reinforcement along with constructive criticism can significantly enhance their service delivery, including monitoring progress and reporting on serious and non-serious adverse events, ultimately benefiting the overall project outcomes.
- Celebrate Milestones: Together, acknowledge and celebrate project milestones and successes. Recognizing these achievements not only strengthens the partnership but also serves as motivation for both teams to continue striving for excellence.
- Address Issues Promptly: When challenges arise, tackle them swiftly and collaboratively. Maintaining an open dialogue about issues often leads to effective solutions and helps prevent escalation. This practice is backed by results from a recent investigation that underscored the need for additional research on communication behaviors in healthcare partnerships, stressing the significance of addressing issues swiftly.
- Invest in Relationship Development: Actively seek opportunities for team-building activities or joint training sessions to foster rapport and deepen understanding between your team and the CRO. Such investments in relationship development can lead to improved collaboration and project success.
- Emphasize Regulatory Compliance: Ensure that both parties understand the importance of compliance with country requirements, including obtaining necessary import permits and nationalization of investigational devices. Understanding how to choose the right CRO for your clinical trials is crucial for their success and helps mitigate risks associated with regulatory issues. Incorporating these strategies into your partnership approach can significantly enhance the effectiveness of CRO collaborations, ultimately contributing to the success of clinical trials. As Johnson and Bradbury noted, the impact of effective communication in partnerships cannot be overstated.
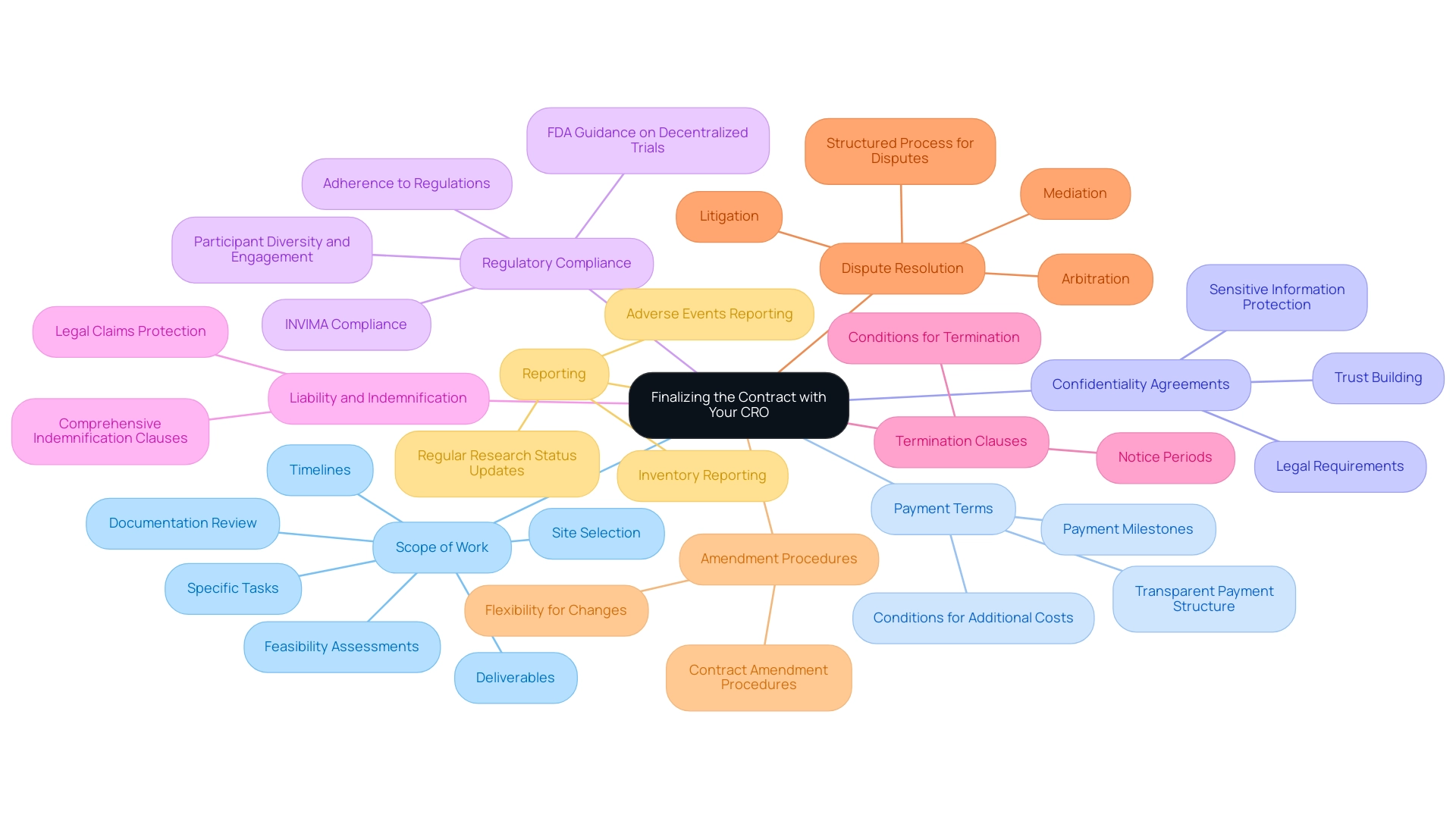
Finalizing the Contract with Your CRO
When finalizing a contract with your Contract Research Organization (CRO), it is crucial to include several key elements to ensure a successful partnership:
-
Scope of Work: Clearly defining the scope of services is paramount. This should encompass specific tasks, deliverables, and timelines, which are essential for maintaining clarity throughout the project.
CRO services often encompass feasibility assessments, site selection, and review and feedback on documentation to ensure adherence to country requirements, guaranteeing that the research is carried out in compliant and optimal environments.
-
Payment Terms: Establishing a transparent payment structure is vital. Outline the payment milestones and any conditions that may lead to additional costs, ensuring both parties have aligned expectations.
-
Confidentiality Agreements: It is essential to include clauses that safeguard sensitive information and maintain confidentiality throughout the research. This is not only a legal requirement but also fosters trust between the parties involved.
-
Regulatory Compliance: The contract should explicitly state the CRO's obligation to adhere to all relevant regulations and guidelines, such as those enforced by INVIMA, Colombia's Level 4 health authority.
With the FDA's Draft Guidance promoting decentralized trials, ensuring compliance is more critical than ever, particularly in enhancing participant diversity and engagement.
-
Liability and Indemnification: Addressing liability issues through comprehensive indemnification clauses is necessary to protect both parties from potential legal claims, ensuring a secure operational environment.
-
Termination Clauses: Clearly specifying conditions for termination, along with required notice periods, allows for flexibility and clear exit strategies should the need arise.
-
Dispute Resolution: Including a structured process for managing disputes—whether through mediation, arbitration, or litigation—can save both time and resources, allowing for swift resolutions to conflicts that may occur.
-
Amendment Procedures: Outlining the procedures for contract amendments is crucial. This guarantees flexibility to unexpected alterations in the research, illustrating the dynamic nature of medical investigation.
-
Reporting: It is essential to include provisions for regular reporting on research status, inventory, and serious and non-serious adverse events, ensuring transparency and accountability throughout the trial.
By meticulously defining and agreeing upon these elements, you lay a solid foundation for a productive collaboration with your CRO, which is critical when considering how to choose the right CRO for your clinical trials, ultimately contributing to the success of your clinical research initiatives.
For instance, a recent case study highlighted how a major pharmaceutical company successfully negotiated their CRO contract by emphasizing clear payment terms and regulatory compliance, leading to a 30% reduction in customer support costs through improved operational efficiency, akin to the benefits seen with AI chatbots.
As Maria Harutyunyan, an expert in Regulatory Affairs, aptly stated, 'Want to know what are the best SEO companies for the Atlanta market?'—this reflects the importance of knowing how to choose the right CRO for your clinical trials partner, much like choosing the right SEO firm.
Conclusion
Selecting the right Clinical Research Organization (CRO) is critical to the success of clinical trials, particularly in the complex landscape of first-in-human medical devices in Colombia. Evaluating key factors such as:
- Expertise
- Regulatory compliance
- Technological capabilities
is essential in making an informed decision. A systematic approach—starting from defining specific needs to assessing performance metrics—ensures that organizations choose a CRO aligned with their objectives and capable of navigating the intricacies of clinical research.
Moreover, fostering a strong partnership with the selected CRO enhances collaboration and communication, which are vital for overcoming challenges and achieving project milestones. Clear expectations, regular updates, and constructive feedback create an environment conducive to success. Finalizing a comprehensive contract that outlines:
- The scope of work
- Payment terms
- Confidentiality agreements
- Compliance obligations
lays the groundwork for a productive relationship.
In conclusion, the careful selection and management of a CRO can significantly influence the outcomes of clinical trials. By prioritizing the right criteria and nurturing a collaborative environment, organizations can not only improve their chances of success but also contribute positively to the advancements in medical technology and patient care in Colombia. Emphasizing diligence and foresight in this process will ultimately lead to more efficient and compliant clinical research endeavors.
Frequently Asked Questions
What key factors should be prioritized when choosing a Clinical Research Organization (CRO) for first-in-human medical devices in Colombia?
Key factors include expertise and experience, regulatory compliance, services offered, technology and infrastructure, team qualifications, communication and collaboration, and cost and budgeting.
Why is expertise and experience important when selecting a CRO?
Expertise and experience are crucial because organizations with a proven track record in managing clinical trials relevant to your therapeutic area are more likely to meet the unique demands of your research.
What role does regulatory compliance play in selecting a CRO?
Regulatory compliance ensures that the CRO adheres to all relevant regulations, including Good Clinical Practice (GCP) guidelines, which is essential for maintaining the integrity of the research and safeguarding patient welfare.
What services should a full-service CRO provide?
A full-service CRO should provide comprehensive support, including research design, protocol writing, data analysis, feasibility studies, site selection, project management, and the management of clinical study agreements and import permits.
How can technology and infrastructure impact the selection of a CRO?
The CRO's technological capabilities, such as data management systems and electronic data capture tools, can enhance efficiency and accuracy in data collection and reporting, which is vital for the success of the study.
What qualifications should I look for in the CRO's team?
It is important to ensure that the CRO's personnel possess the necessary expertise and qualifications to effectively manage your research.
Why is communication and collaboration important in a CRO partnership?
Effective communication fosters a successful partnership, making it essential to assess the CRO’s engagement approach and responsiveness to inquiries and needs.
How should I approach budgeting when selecting a CRO?
Understand the CRO's pricing structure and ensure that the services provided align with your budget while maintaining high quality standards.
What steps should I follow to choose the right CRO for my clinical trials?
Steps include defining your needs, conducting preliminary research, requesting proposals, evaluating proposals, scheduling interviews, checking references, making a decision, and negotiating contract terms.
What should I do after selecting a CRO?
After selecting a CRO, engage in negotiations regarding contract terms to clarify deliverables, timelines, and payment structures, which is crucial for a productive working relationship.
What risks are associated with changing a CRO mid-study?
Changing a CRO mid-study can introduce significant technical and financial risks, highlighting the importance of making the right choice from the outset.




