Overview
Choosing the right First-in-Human Studies CRO involves assessing the unique needs of your study, evaluating the CRO's expertise and past performance, and navigating regulatory considerations effectively. The article outlines a systematic approach that emphasizes the importance of clear objectives, collaboration with local stakeholders, and the establishment of performance metrics to ensure the success and compliance of clinical trials.
Introduction
In the realm of clinical research, the initiation of first-in-human (FIH) studies represents a critical juncture in the development of innovative therapies. These trials not only pave the way for evaluating the safety and efficacy of new drug candidates but also require meticulous planning and execution.
A comprehensive understanding of the unique needs and requirements of each study is paramount, encompassing everything from regulatory considerations to effective stakeholder engagement.
As organizations navigate this complex landscape, the selection of a capable Contract Research Organization (CRO) becomes essential, ensuring that trials are conducted efficiently and in compliance with both local and international standards.
This article delves into the multifaceted aspects of conducting FIH studies, highlighting the importance of strategic planning, collaboration, and performance assessment in achieving successful outcomes.
Identifying Your Study's Unique Needs and Requirements
Establishing clear objectives for your First-in-Human Studies CRO trial is crucial. Begin by analyzing the target population demographics, as this will influence selection criteria and recruitment strategies. Additionally, examine the research design meticulously, focusing on specific endpoints that align with regulatory expectations.
It's essential to note that a single-dose extended toxicity examination is the minimal requirement to perform Phase 0 trials in humans, highlighting the need for thorough preparation. The complexity of interventions must also be assessed, along with any unique methodological requirements relevant to your research. For instance, special populations or specific conditions may necessitate tailored approaches to ensure safety and efficacy.
A comprehensive safety monitoring plan should be established beforehand, detailing data collection and review processes to mitigate risks effectively. This essential knowledge is crucial for recognizing a First-in-Human Studies CRO, such as bioaccess®, which provides expedited medical device research services in Latin America and has the expertise needed to meet these particular needs. The achievement of First-in-Human Studies CRO research depends on a cross-disciplinary group of clinicians, statisticians, regulatory experts, and other professionals, which highlights the cooperative essence of these experiments.
Alongside First-in-Human Studies CRO research, bioaccess® focuses on managing Early-Feasibility Assessments (EFA), Pilot Research, and Post-Market Follow-Up Evaluations (PMCF), ensuring a thorough approach to management. First-in-Human Studies CRO investigations provide the initial opportunity to assess the immunogenicity potential of new biologic drug candidates, which is a crucial element of safety and effectiveness. However, the immunogenicity profiles observed in FIH studies may not accurately reflect those of the target patient population, indicating the need for careful interpretation.
As Dr. Pui Man Leung, a key member of the BDD team, emphasizes, 'His expertise and forward-thinking approach are integral to the development of novel therapies and advancements in the field.' This statement highlights the significance of defining research objectives with precision to navigate the complexities inherent in FIH trials successfully, while leveraging bioaccess®'s comprehensive clinical trial management services, including feasibility assessments, site selection, compliance reviews, and project management. Their customized approach allows for flexibility in navigating the regulatory environments, ensuring that each project is tailored to meet both scientific and regulatory standards.
Evaluating CRO Expertise and Capabilities for First-in-Human Studies
When selecting a First-in-Human Studies CRO for first-in-human trials, it is essential to thoroughly assess their past performance in this area. This includes examining the types of products they have successfully handled and the outcomes of those studies. For instance, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for research studies in Latin America, backed by the Colombian Minister of Health.
Notably, Smietana et al. examined the success of over 9,200 new compounds from 1996 to 2014, emphasizing the significance of choosing CROs with established track records. The partnership has achieved significant milestones, including a reported 50% reduction in recruitment time and retention rates of 95%, showcasing the effectiveness of their collaboration.
Prioritize First-in-Human Studies CROs that feature specialized teams with a deep understanding of the intricacies associated with early-phase clinical studies. Client testimonials, like those from Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, who shared his positive experience with bioaccess® during its First-in-Human Studies CRO in Colombia, can offer valuable insights into their success in similar scenarios. Moreover, the challenges encountered by Class II drug candidates, which frequently necessitate high doses to attain efficacy, highlight the importance for CROs to possess experience in managing complex studies.
Evaluating operational capabilities is similarly crucial; this entails examining project coordination approaches, quality assurance procedures, and the technological tools that enable effective execution. For instance, the use of advanced data management systems can enhance real-time monitoring and streamline communication among stakeholders. As Asher Mullard noted, McKinsey calculated an above-average overall success rate for rare disease drugs, hitting 29% over the past three years, emphasizing the need for experienced partners in the research endeavor.
Such a thorough assessment not only helps in choosing a competent CRO but also corresponds with results that suggest quality and speed are linked to research success.
Navigating Regulatory Considerations in First-in-Human Studies
Navigating the regulatory guidelines for first-in-human trials in Latin America requires a thorough understanding of local requirements and international standards. Collaborating with a First-in-Human Studies CRO, such as bioaccess®, can improve your project's success by utilizing their over 20 years of experience in medical device clinical research. Their extensive services encompass:
- Viability assessments
- Site selection
- Compliance evaluations
- Experimental setup
- Project management
With a specialized emphasis on Early-Feasibility Assessments (EFA) and the First-in-Human Studies CRO.
It is crucial to inquire about their specific experience with the Investigational New Drug (IND) application process and obtaining ethical approvals, as a First-in-Human Studies CRO with strong regulatory expertise can significantly streamline compliance efforts. Recent trends indicate that adherence to established guidelines not only facilitates smoother study initiation but also increases the likelihood of successful regulatory submissions. By partnering with a CRO that comprehends these complexities and provides a tailored strategy for managing research processes, you can enhance your project's chances for both regulatory approval and operational success in the competitive environment of medical device evaluations.
Building Collaborations with Local Stakeholders for Success
Recognizing and involving important local stakeholders—such as healthcare providers, regulatory bodies, and patient advocacy organizations—is essential for the success of research studies in Latin America. Our comprehensive clinical trial management services, which include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
underscore the importance of early involvement of these stakeholders. This proactive engagement fosters trust and enhances communication throughout the research process, contributing to five key impacts:
- User-centeredness and acceptability
- Feasibility
- Quality
- Engagement scope and quality
- Relevance
Establishing advisory boards consisting of local experts can provide valuable insights into community needs and preferences, leading to more relevant and effective study designs. For instance, updates to the Project Management Plan reflect changes in stakeholder management strategies, ensuring that the plan remains effective in controlling stakeholder engagement. The roles of ethics committees and health ministries are also vital during the trial setup process to ensure compliance with local regulations.
This collaborative strategy not only enriches the research framework but also significantly improves participant recruitment and retention rates; research indicates that effective local stakeholder engagement can enhance recruitment rates by up to 30% in First-in-Human Studies CRO, ultimately contributing to the overall success of early-feasibility initiatives. Additionally, our reporting mechanisms ensure regular updates on research status and adverse events, further supporting the integrity and transparency of the research process.
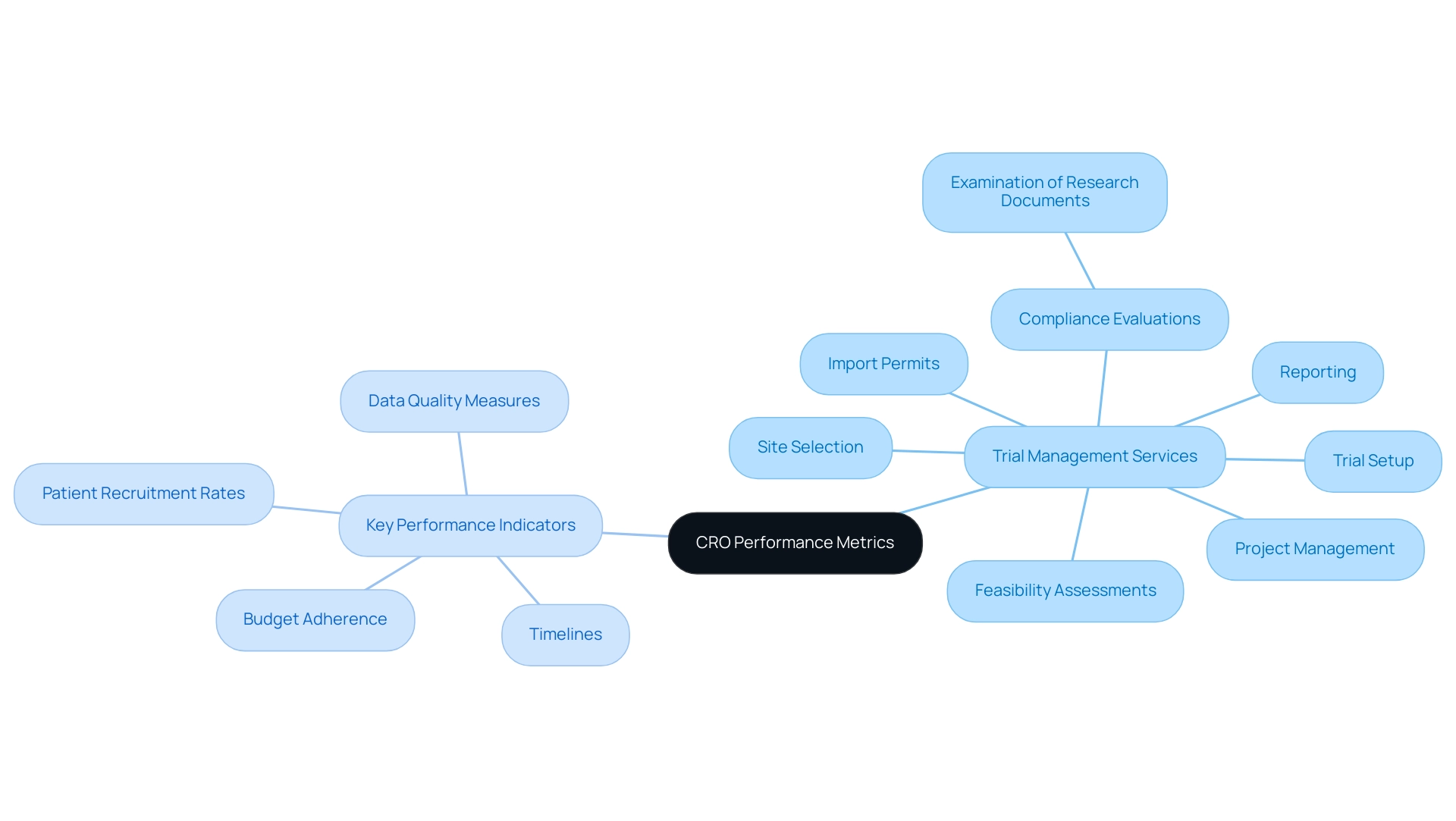
Assessing CRO Performance and Success Metrics
Setting clear performance metrics from the beginning is essential for the success of any research, especially concerning stringent ethical and safety standards that regulate these investigations. Our extensive trial management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations—including the examination of research documents to meet national requirements
- Trial setup
- Import permits
- Project management
- Reporting
All of which play a crucial role in achieving these metrics. Key performance indicators should encompass:
- Timelines
- Budget adherence
- Patient recruitment rates
- Data quality measures
While consistently prioritizing participant safety.
A recent analysis indicates that as of May 2023, 94% of interventional research projects have successfully published their results, highlighting the importance of these metrics in achieving research objectives. Furthermore, with the recent merger of two clinical research firms in December 2023, there is a renewed focus on enhancing performance metrics across various therapeutic areas. Regularly reviewing these indicators with the First-in-Human Studies CRO throughout the study ensures that all parties stay aligned with the overarching goals.
Implementing a feedback loop is essential to allow for timely adjustments based on performance evaluations, particularly in relation to project management and compliance. As Rowena Young from the Skoll Foundation aptly states, 'The risk with any metric is that people will come to see it as a description of reality, rather than a tool for a conversation about reality.' This proactive approach not only promotes accountability among stakeholders but also significantly improves the quality and results of the research study.
Additionally, understanding recent trends in R&D funding and deal activity can provide valuable insights into the changing market conditions that influence clinical trial performance metrics.
Conclusion
The successful execution of first-in-human (FIH) studies hinges on a multifaceted approach that encompasses a variety of essential components. Identifying the unique needs and requirements of each study is a foundational step, which includes:
- Setting clear objectives
- Analyzing target demographics
- Establishing robust safety monitoring plans
The collaboration with a capable Contract Research Organization (CRO) is crucial, as their expertise can significantly influence the trial’s success by ensuring compliance with regulatory standards and expediting the clinical trial process.
Evaluating the capabilities and historical performance of potential CROs is equally important. Organizations with proven track records, specialized teams, and advanced project management methodologies are more likely to navigate the complexities of early-phase trials effectively. Furthermore, engaging local stakeholders throughout the clinical trial process fosters trust and enhances communication, which can lead to improved participant recruitment and retention rates.
Ultimately, establishing clear performance metrics and maintaining an adaptive approach to project management can help ensure that FIH studies meet their objectives efficiently. By leveraging the expertise of experienced partners and fostering collaborative relationships, organizations can enhance their chances of success in the competitive landscape of clinical research. The journey of bringing innovative therapies to market begins with meticulous planning and execution, underscoring the critical nature of first-in-human studies in the development of new medical advancements.
Frequently Asked Questions
Why is it important to establish clear objectives for First-in-Human Studies CRO trials?
Establishing clear objectives is crucial as it influences the selection criteria, recruitment strategies, and overall research design, ensuring alignment with regulatory expectations.
What is the minimal requirement for conducting Phase 0 trials in humans?
A single-dose extended toxicity examination is the minimal requirement to perform Phase 0 trials in humans.
What factors should be assessed when planning First-in-Human Studies?
Factors include the complexity of interventions, unique methodological requirements, and the need for tailored approaches for special populations or specific conditions.
What is a comprehensive safety monitoring plan, and why is it necessary?
A comprehensive safety monitoring plan outlines data collection and review processes to effectively mitigate risks, ensuring participant safety during trials.
What services does bioaccess® provide in the context of First-in-Human Studies?
Bioaccess® offers expedited medical device research services, Early-Feasibility Assessments, Pilot Research, and Post-Market Follow-Up Evaluations, focusing on comprehensive clinical trial management.
What is the significance of immunogenicity assessments in First-in-Human Studies?
These assessments provide the initial opportunity to evaluate the safety and effectiveness of new biologic drug candidates, though the results may not always reflect the target patient population.
How can past performance of a CRO influence the selection process for First-in-Human trials?
Evaluating a CRO's past performance helps determine their experience with similar products and the outcomes of those studies, which is crucial for informed decision-making.
What achievements have resulted from the partnership between bioaccess™ and Caribbean Health Group?
This partnership has led to significant milestones, including a reported 50% reduction in recruitment time and retention rates of 95%.
What should be prioritized when selecting a First-in-Human Studies CRO?
It is essential to prioritize CROs with specialized teams that understand the intricacies of early-phase clinical studies and have a proven track record.
Why is evaluating operational capabilities important in choosing a CRO?
Assessing operational capabilities, such as project coordination, quality assurance, and technological tools, is vital for ensuring effective execution of clinical trials.
What does research suggest about the relationship between quality, speed, and research success?
Results indicate that there is a link between quality and speed in the research process, which correlates with overall research success.

