Introduction
The medical device Contract Research Organization (CRO) market is experiencing unprecedented growth, driven by advancements in technology and increasing regulatory demands. As organizations seek to navigate this complex landscape, understanding the pivotal factors influencing CRO selection becomes essential.
This article delves into the intricacies of the medical device CRO market, highlighting key trends, regional insights, and strategic considerations for companies looking to optimize their clinical trial processes. From evaluating regulatory expertise to recognizing the importance of local market knowledge, the insights provided will equip stakeholders with the knowledge needed to make informed decisions in a rapidly evolving environment.
Overview of the Medical Device CRO Market Landscape
The growth of the medical device CRO by country market is significant, primarily driven by technological advancements and increased compliance requirements. As of 2024, this global market is projected to expand significantly, with the regulatory and medical affairs segment anticipated to lead this growth at a compound annual growth rate (CAGR) of 12.4%. Key players in this space include Avania, Charles River Laboratories, CROMSOURCE, and others, each contributing unique capabilities and specializations.
A significant trend influencing the landscape is the growing movement towards outsourcing research studies to areas like Latin America, where partnerships such as that between bioaccess™ and Caribbean Health Group strive to establish Barranquilla as a premier destination for medical research, backed by Colombia's Minister of Health. Furthermore, Flow-FX's first-in-human study of its innovative Flow-Screw instrument for intraosseous antibiotic delivery is being carried out in Colombia, emphasizing the region's potential. GlobalCare Clinical Studies has also collaborated with bioaccess™ to improve ambulatory services for studies, achieving over a 50% reduction in recruitment time and a 95% retention rate.
When evaluating a Medical Device CRO by Country, organizations must carefully consider several factors, including its size, expertise in specific medical devices, and a proven track record of successful project delivery. For instance, the acquisition of SAMDI Tech by Charles River Laboratories in January 2023 exemplifies how established players are enhancing their portfolios to meet these demands. By thoroughly evaluating these components, companies can strategically position themselves to leverage the strengths of the right CRO, ultimately fostering more efficient and compliant research processes.
bioaccess™ offers comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Katherine Ruiz, a compliance affairs expert, enhances bioaccess™'s credibility as a partner for U.S. medical equipment companies, bringing valuable insights from her experience at Colombia's oversight agency, INVIMA.
Key Factors in Choosing a Medical Device CRO by Country
Choosing a Medical Device CRO by Country requires thorough evaluation of various essential factors, particularly compliance expertise, local market insight, and language skills. Each nation functions within a unique governance framework that significantly impacts the medical device approval process. In Colombia, for example, INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) plays a crucial role as the national authority, overseeing the marketing and manufacturing of health products and ensuring adherence to safety and efficacy standards.
Their designation as a Level 4 health authority by the Pan American Health Organization/World Health Organization highlights their competence in oversight functions. Present legal challenges can differ by nation and greatly influence timelines and success rates. Consequently, it's crucial to assess the CRO's knowledge of INVIMA's compliance framework, along with their history in managing these intricacies.
Furthermore, the CRO's experience with particular product types and their awareness of local clinical practices are important. Katherine Ruiz, a Regulatory Affairs expert with extensive experience in obtaining market clearance for medical products in Colombia, exemplifies the level of expertise that can enhance a CRO’s capabilities. In areas like the Asia Pacific, which represented 41.9% of the Medical Device CRO by Country market in 2023, the capacity to adjust to local needs is essential.
The strong growth prospects in this area, as highlighted in the case study titled 'Asia Pacific Market Dominance,' indicate an increasing number of Medical Device CRO by Country, making local knowledge a key asset. Moreover, as indicated by the Argentine Chamber of Medical Specialties (CAEME), 'Travel expenses must be reasonable and limited to the days on which the scientific or professional event is planned to be held,' highlighting the significance of understanding local practices and regulations when choosing a CRO.
A thorough assessment of the CRO's service capabilities, including:
- feasibility studies
- site selection
- study setup
- import permits
- project management
- reporting
is essential for the success of your project. Assessing their resources, including access to diverse patient populations and robust technology infrastructure, will ensure that the chosen CRO is well-aligned with your project goals, thereby enhancing the likelihood of a successful collaboration.
Regional Insights: Trends in Medical Device CROs Across the Globe
In the United States, a significant trend is emerging towards the integration of digital health within research studies, with Contract Research Organizations (CROss) increasingly leveraging innovative technologies to enhance operational efficiency and streamline research processes. These advancements are crucial in shortening study timelines and enhancing data precision, especially in the context of Latin America, where Medtech companies encounter numerous challenges such as regulatory obstacles, language barriers, limited resources, and the fragmentation of research resources. As indicated in recent evaluations, the economic effect of digital health integration is significant, with an anticipated rise in efficiency resulting in cost reductions of up to 30% in research operations.
Additionally, the partnership between Greenlight Guru and bioaccess™ seeks to close these gaps by enabling early-stage studies in Latin America, demonstrated by PAVmed's successful first-in-human research in Colombia. This partnership is vital in tackling the need for seamless communication and collaboration between American clinical trial clients and Latin American hospitals, which frequently encounter challenges due to differing professional standards and legal environments. Simultaneously, compliance harmonization efforts across Europe are reshaping the CRO landscape, necessitating that companies partner with organizations skilled in navigating both EU-wide regulations and local compliance requirements.
As Klaus F. Zimmermann & Anzelika Zaiceva emphasize in their work on labor migration, 'comprehending the scale, diversity, and factors of governance environments is crucial for organizations functioning within these frameworks.' This alignment is essential as stakeholders must act swiftly to adjust to the changing compliance framework to ensure lawful operations. Furthermore, the Asia Pacific area is undergoing swift expansion in research activities, propelled by heightened investments in healthcare infrastructure and a rising need for research studies.
Drawing parallels with the case study on household constraints and dysfunctional rural-urban migration, CROss face similar challenges in adapting to regulatory environments, emphasizing the importance of strategic decision-making. Grasping these regional dynamics is essential for organizations to make informed strategic choices about their research sites and the selection of Medical Device CRO by Country partners, thereby enhancing their study outcomes and contributing to job creation, economic growth, and healthcare advancement in local economies. Comprehensive research management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting, play a critical role in navigating these challenges and ensuring successful outcomes.
Top Medical Device CROs: Who to Consider for Your Projects
In the realm of Medical Device CRO by Country, established names such as Medpace, Parexel, and Covance are recognized for their commitment to high-quality services across various therapeutic areas. However, specialized companies like bioaccess® offer unique knowledge in managing research studies specifically in Latin America, particularly in the context of Medical Device CRO by Country, focusing on critical study types such as:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
The global CRO market is anticipated to attain 108 billion U.S. dollars by 2032, making it essential for organizations to collaborate with reputable CROs to enhance the credibility of their research.
A partnership with bioaccess® not only provides access to extensive resources and networks vital for successful project outcomes but also guarantees adherence to local regulations through their comprehensive services—including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
This is particularly important as the biopharma outsourcing model evolves from 2022 to 2024, highlighting the necessity of specialized Medical Device CRO by Country to address unique project requirements. For example, the recent collaboration between Avance Clinical and Right demonstrates how innovative technologies can improve operational efficiency in research studies.
Moreover, industry specialist Matej Mikulic's statement to 'Contact us now' highlights the urgency for organizations to connect with specialized and reputable CROs like bioaccess®, particularly as the oncology sector expands, propelled by precision medicine and its rising need for comprehensive data gathering and research support. By engaging in research studies, companies not only advance their research but also contribute to local economies through job creation, economic growth, and improved healthcare outcomes. Don't miss the opportunity to collaborate with bioaccess®; contact us today to discover how we can assist with your clinical study requirements.
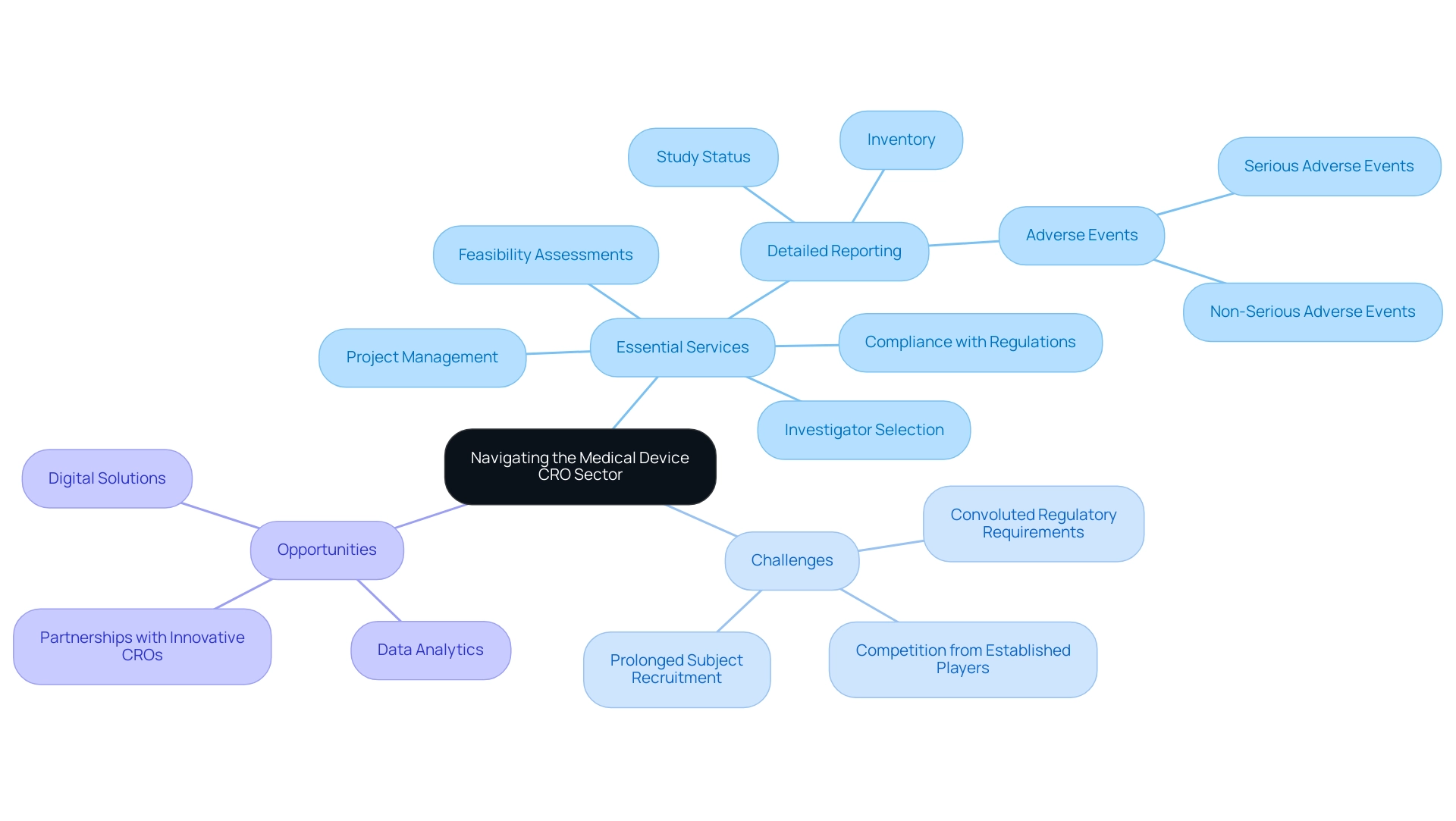
Navigating Challenges and Opportunities in the Medical Device CRO Sector
The medical equipment CRO sector is presently maneuvering through a terrain characterized by rising oversight, escalating expenses, and the need for faster turnaround times. Within this context, our extensive process for progressing medical device studies includes essential services such as:
- Feasibility assessments
- Investigator selection
- Compliance with regulations
- Project management
- Detailed reporting (including study status, inventory, and serious and non-serious adverse events)
In countries like China, health insurance coverage has expanded to over 95% of the population, revealing significant market dynamics and opportunities, particularly in the Asia Pacific region.
However, challenges such as:
- Convoluted regulatory requirements
- Competition from established players
- Prolonged subject recruitment
necessitate a strategic reassessment. Firms that focus on investments in digital solutions and data analytics can improve operational efficiency and simplify research processes, thereby gaining a competitive advantage. Furthermore, fostering robust partnerships with innovative CROs that demonstrate adaptability is essential for effectively managing the complexities of clinical research.
Customized solutions are essential for tackling these challenges, ensuring that startups can navigate the complex terrain of medical trials. The Asia Pacific market, which is a key player in the Medical Device CRO by Country with a revenue share of 41.9% in 2023, illustrates the success driven by improvements in the regulatory framework and a growing number of research organizations. By comprehensively understanding these dynamics, organizations can strategically position themselves to capitalize on emerging trends while adeptly mitigating associated risks, ultimately driving global health improvement through international collaboration and innovation in Medtech.
Conclusion
The medical device Contract Research Organization (CRO) market is poised for significant transformation, driven by technological advancements and increasing regulatory complexity. As highlighted, the importance of selecting the right CRO cannot be overstated; it involves evaluating critical factors such as:
- Regulatory expertise
- Local market knowledge
- The ability to navigate diverse regulatory landscapes
With regions like Latin America emerging as key players, understanding local practices and regulatory frameworks becomes paramount for successful clinical trials.
Furthermore, the integration of digital health solutions is reshaping clinical trial methodologies, enhancing operational efficiency, and ultimately reducing costs. The collaboration between established CROs and specialized firms, such as bioaccess®, showcases the potential for improved outcomes through tailored services and localized expertise. As the market continues to evolve, organizations must remain agile, adapting to shifting regulatory environments while leveraging the strengths of their CRO partners to optimize clinical trial processes.
In conclusion, navigating the complexities of the medical device CRO market requires a strategic and informed approach. By prioritizing partnerships with CROs that demonstrate both local knowledge and a robust service offering, stakeholders can enhance their chances of successful project delivery. As the sector continues to grow, staying attuned to emerging trends and regulatory developments will be essential for driving innovation and improving healthcare outcomes globally.
Frequently Asked Questions
What is driving the growth of the medical device CRO market?
The growth of the medical device CRO market is primarily driven by technological advancements and increased compliance requirements, with a projected compound annual growth rate (CAGR) of 12.4% in the regulatory and medical affairs segment.
Who are the key players in the medical device CRO market?
Key players in the medical device CRO market include Avania, Charles River Laboratories, and CROMSOURCE, among others, each offering unique capabilities and specializations.
What trends are influencing the medical device CRO landscape?
A significant trend is the outsourcing of research studies to regions like Latin America, with partnerships such as bioaccess™ and Caribbean Health Group aiming to establish Barranquilla as a key medical research destination.
What services does bioaccess™ offer for clinical trial management?
bioaccess™ offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What factors should organizations consider when evaluating a Medical Device CRO by Country?
Organizations should consider factors such as the CRO's size, expertise in specific medical devices, proven track record of successful project delivery, compliance expertise, local market insight, and language skills.
What role does INVIMA play in Colombia's medical device approval process?
INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) is the national authority in Colombia overseeing the marketing and manufacturing of health products, ensuring adherence to safety and efficacy standards.
How does digital health integration impact research studies in the United States?
Digital health integration enhances operational efficiency and streamlines research processes, leading to cost reductions of up to 30% in research operations.
What is the significance of local knowledge for Medical Device CROs in the Asia Pacific region?
Local knowledge is essential for adapting to regulatory environments and meeting the unique needs of the region, which represented 41.9% of the Medical Device CRO market in 2023.
What types of studies do specialized CROs like bioaccess® focus on?
Specialized CROs like bioaccess® focus on critical study types such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
What challenges are faced by the medical device CRO sector?
Challenges include rising oversight, escalating expenses, convoluted regulatory requirements, competition from established players, and prolonged subject recruitment.
How can firms improve operational efficiency in clinical research?
Firms can improve operational efficiency by investing in digital solutions and data analytics, as well as fostering partnerships with innovative CROs that demonstrate adaptability.

