Introduction
Navigating the complex landscape of clinical research for medical devices requires a strategic approach, particularly when considering the role of Contract Research Organizations (CROs). These specialized entities offer critical support to Medtech startups, addressing challenges such as:
- Regulatory compliance
- Communication barriers
- The need for efficient trial management
As the demand for innovative medical solutions grows, understanding the selection criteria for a suitable CRO becomes paramount. This article delves into the essential factors that stakeholders must evaluate to ensure successful clinical trials, highlighting the importance of:
- Expertise
- Regulatory knowledge
- Project management capabilities
in fostering effective partnerships. With a focus on the unique dynamics of the Latin American market, it provides valuable insights into making informed decisions that can significantly impact the trajectory of medical device development.
Understanding the Role of Medical Device CROs in Clinical Research
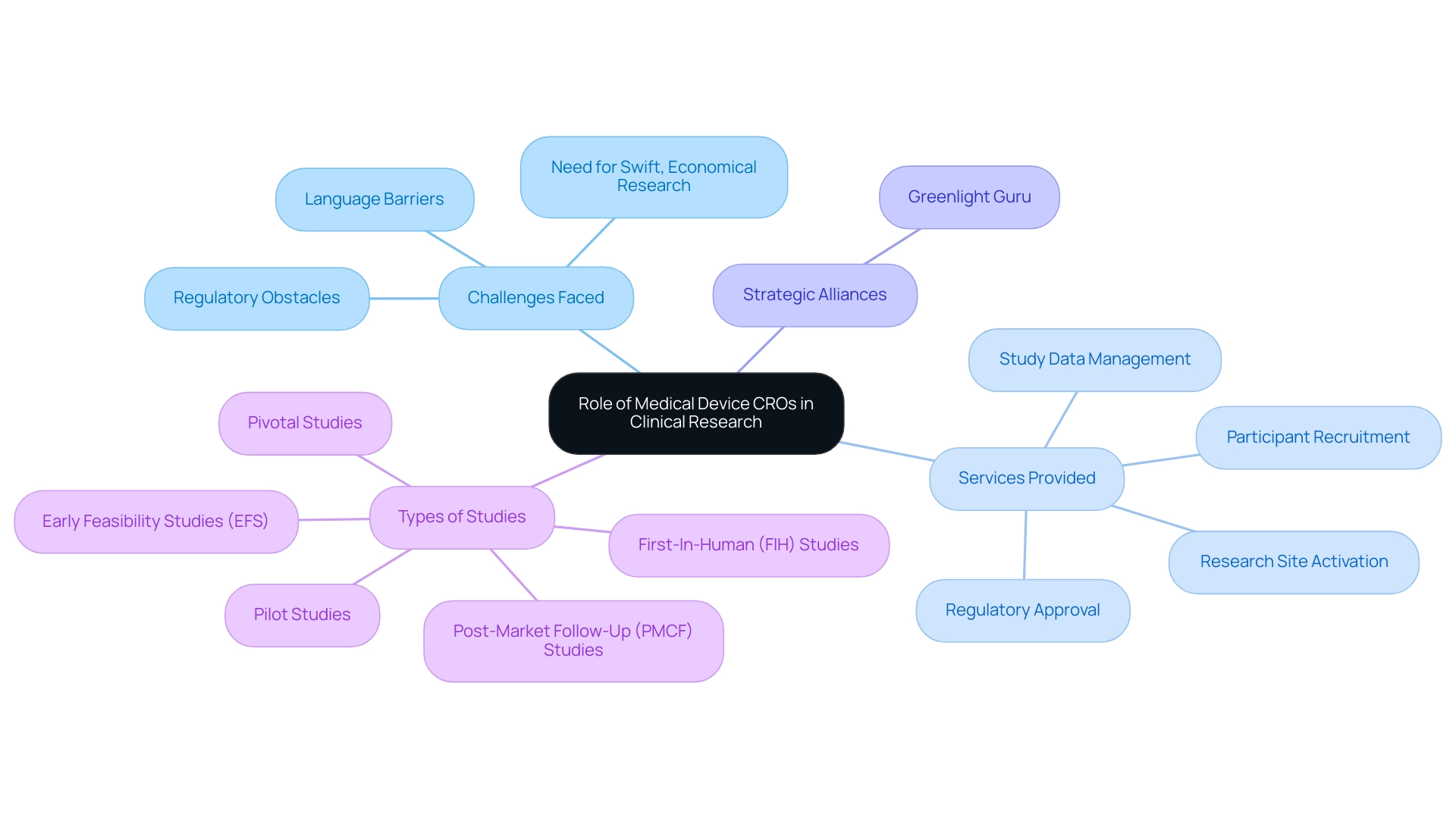
Medical Device CRO Colombia plays a crucial role in the research landscape by providing specialized services that support the development and approval of medical devices. Organizations such as bioaccess®, a prominent Medical Device CRO Colombia, address the distinct challenges faced by Medtech startups, including:
- Regulatory obstacles
- Language barriers
- The necessity for swift, economical research studies
Bioaccess® provides expertise in overseeing research studies, ensuring adherence to regulatory standards, facilitating communication among stakeholders, and offering services such as:
- Regulatory approval
- Research site activation
- Participant recruitment
- Study data management
Their cooperation with Greenlight Guru represents a strategic alliance intended to speed up Medtech innovations and research in the region, underscored by successful initiatives such as PAVmed's first-in-human project in Colombia. Understanding the role of a Medical Device CRO Colombia, such as bioaccess®, is crucial for stakeholders seeking the right partner to navigate the complexities of regulatory requirements and enhance the efficiency of the research process. This is especially urgent as US companies encounter fragmentation of resources in Latin America, making it essential to bridge gaps in research through:
- Early Feasibility Studies (EFS)
- First-In-Human (FIH) studies
- Pilot studies
- Pivotal studies
- Post-Market Follow-Up (PMCF) studies
Key Criteria for Selecting the Right Medical Device CRO
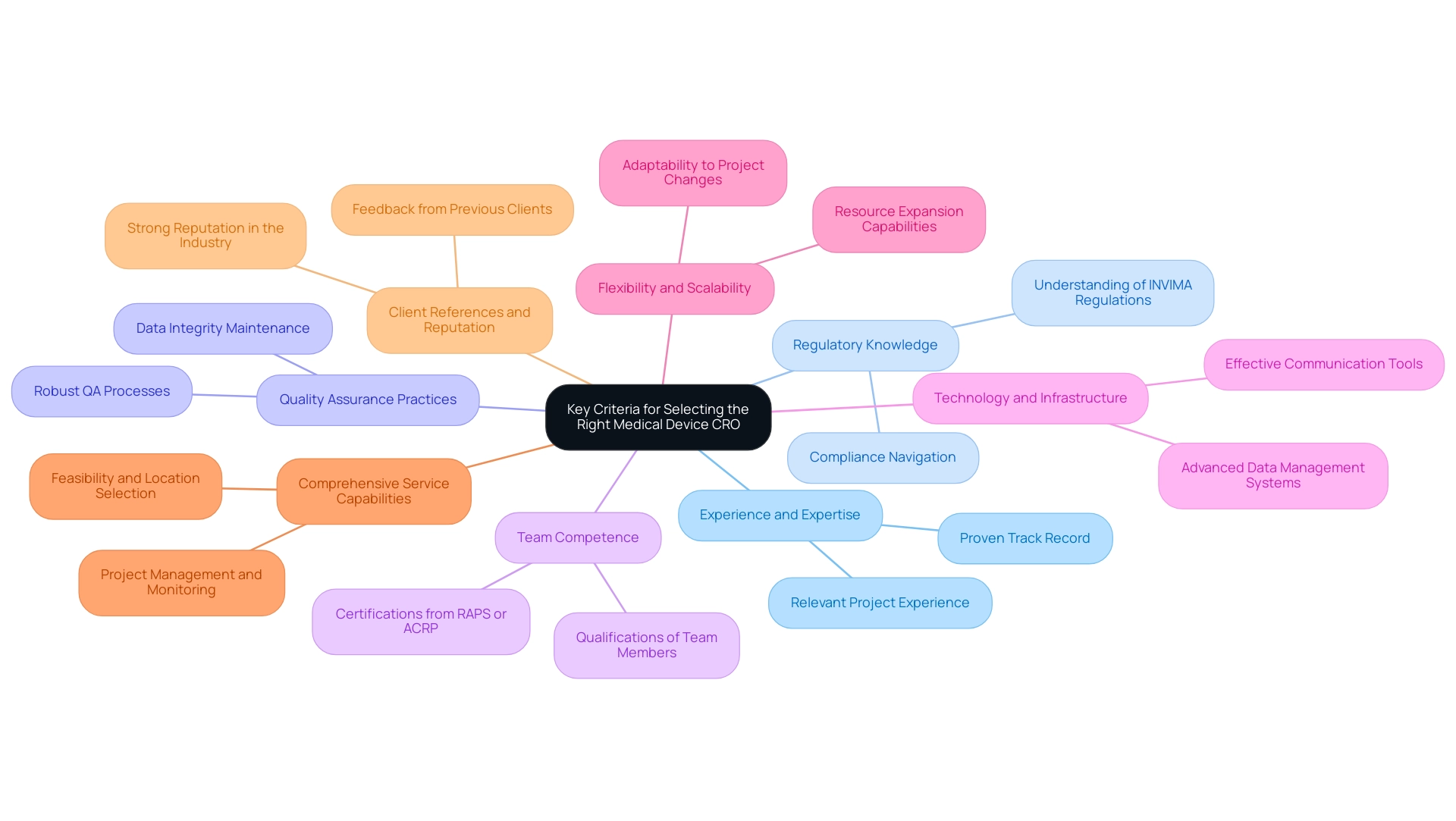
Choosing a Medical Device CRO Colombia necessitates a thorough evaluation of various essential standards to guarantee the success of research studies. The following key factors should be evaluated:
-
Experience and Expertise: It is crucial to evaluate the CRO's background in overseeing studies that correspond with your particular kind of medical device.
A proven track record in similar projects will significantly influence the likelihood of successful outcomes, particularly for Medical Device CRO Colombia in complex environments like Latin America.
-
Regulatory Knowledge: The Medical Device CRO Colombia must possess a deep understanding of both local and international regulatory requirements, particularly those governing medical devices in Colombia, as outlined by INVIMA (Colombia National Food and Drug Surveillance Institute).
This knowledge is vital for navigating the complexities of compliance throughout the assessment process, ensuring adherence to the standards set by INVIMA as a Level 4 health authority recognized by PAHO/WHO.
-
Quality Assurance Practices: Evaluate the CRO's quality management system.
A robust quality assurance (QA) process is crucial for maintaining compliance and ensuring the integrity of the data collected during clinical studies. This is especially significant in the context of medical device research, where regulatory scrutiny can be intense.
-
Team Competence: Review the qualifications and experience of the CRO's team members.
Senior positions in Regulatory Affairs, such as manager or director, typically require a master's degree or higher. Certifications from organizations like RAPS or ACRP can improve career prospects and demonstrate expertise in Regulatory Affairs, reinforcing the credibility of the CRO's team.
Katherine Ruiz, a noted expert in Regulatory Affairs for medical devices in Colombia, exemplifies the level of expertise that can greatly enhance a Medical Device CRO Colombia's effectiveness.
-
Technology and Infrastructure: Consider the technological capabilities and infrastructure of the CRO.
Advanced data management systems and communication tools are crucial for promoting collaboration and ensuring effective project management, especially for initiatives like Early-Feasibility and First-In-Human assessments that require precise execution.
-
Flexibility and Scalability: Assess the CRO’s capacity to adjust to evolving project requirements and expand resources as needed during the different stages of the study.
This adaptability can be crucial in addressing unforeseen challenges that may arise during pivotal research or post-market follow-up evaluations (PMCF).
-
Comprehensive Service Capabilities: Evaluate the CRO's complete array of services, including feasibility and selection of research locations, setup, project management, monitoring, and reporting on project status and adverse events.
Understanding these offerings will help ensure that the CRO can meet all your project needs effectively.
-
Client References and Reputation: Seek feedback from previous clients to assess the CRO's reliability and performance in managing research studies.
A strong reputation and positive client experiences can significantly influence your choice.
As highlighted in the case study on career opportunities in regulatory affairs, expertise in regulatory knowledge can enhance career prospects and the effectiveness of CROs in trials.
By thoroughly evaluating these criteria, stakeholders are better equipped to make informed decisions that align with their project goals, fostering successful outcomes in research.
As Lindus Health appropriately mentions,
Ready to enhance your research experience? Schedule a meeting with our team today and explore how Lindus Health can advance your research, ensuring that your selection of CRO aligns with the extensive support required for successful clinical studies.
Evaluating the CRO’s Project Management Capabilities
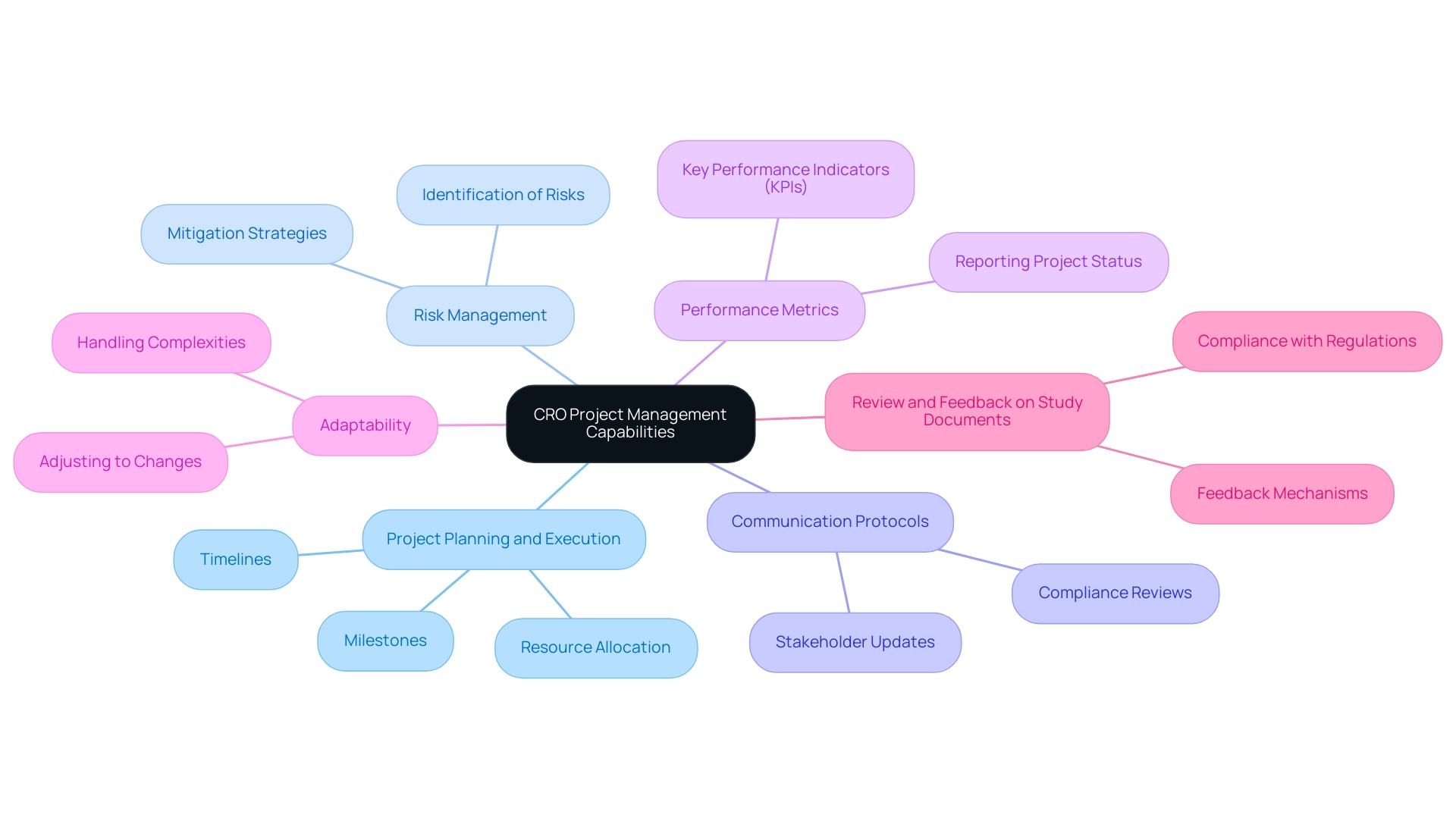
To evaluate a CRO's project management capabilities, it is crucial to consider the following aspects:
-
Project Planning and Execution: Inquire about the CRO's approach to project planning, including timelines, milestones, and resource allocation. A clearly outlined project plan is essential for prompt execution, particularly regarding the conduct of medical device studies in Latin America, as highlighted by Oswaldo Amaya, MD, Clinical Trial Manager at bioaccess™, a prominent Medical Device CRO Colombia.
-
Risk Management: Assess how the CRO identifies and mitigates potential risks throughout the trial phases. Effective risk management strategies, as emphasized by their comprehensive capabilities in project management and monitoring, are essential for maintaining project integrity.
-
Communication Protocols: Understand the communication methods the CRO employs to keep stakeholders informed. Clear and consistent communication is essential for collaboration and transparency, particularly in compliance reviews and feedback on research documents, which are key to meeting country regulations.
-
Performance Metrics: Ask about the key performance indicators (KPIs) the CRO uses to measure project success. Insights into their dedication to quality and accountability can be obtained from their systematic approach to reporting project status, inventory, serious and non-serious adverse events.
-
Adaptability: Assess the CRO's capacity to adjust to unexpected changes or challenges during the testing process. A flexible approach, crucial for navigating complexities like import permits and nationalization of investigational devices, can significantly enhance project outcomes.
-
Review and Feedback on Study Documents: Inquire about the CRO's process for reviewing and providing feedback on study documents to ensure compliance with country requirements. This aspect is essential for upholding regulatory standards and enabling seamless study operations.
By thoroughly evaluating these project management capabilities, stakeholders can ensure they select a CRO that aligns with their operational needs and improves the chances of successful research outcomes.
Assessing the CRO’s Compliance and Regulatory Expertise
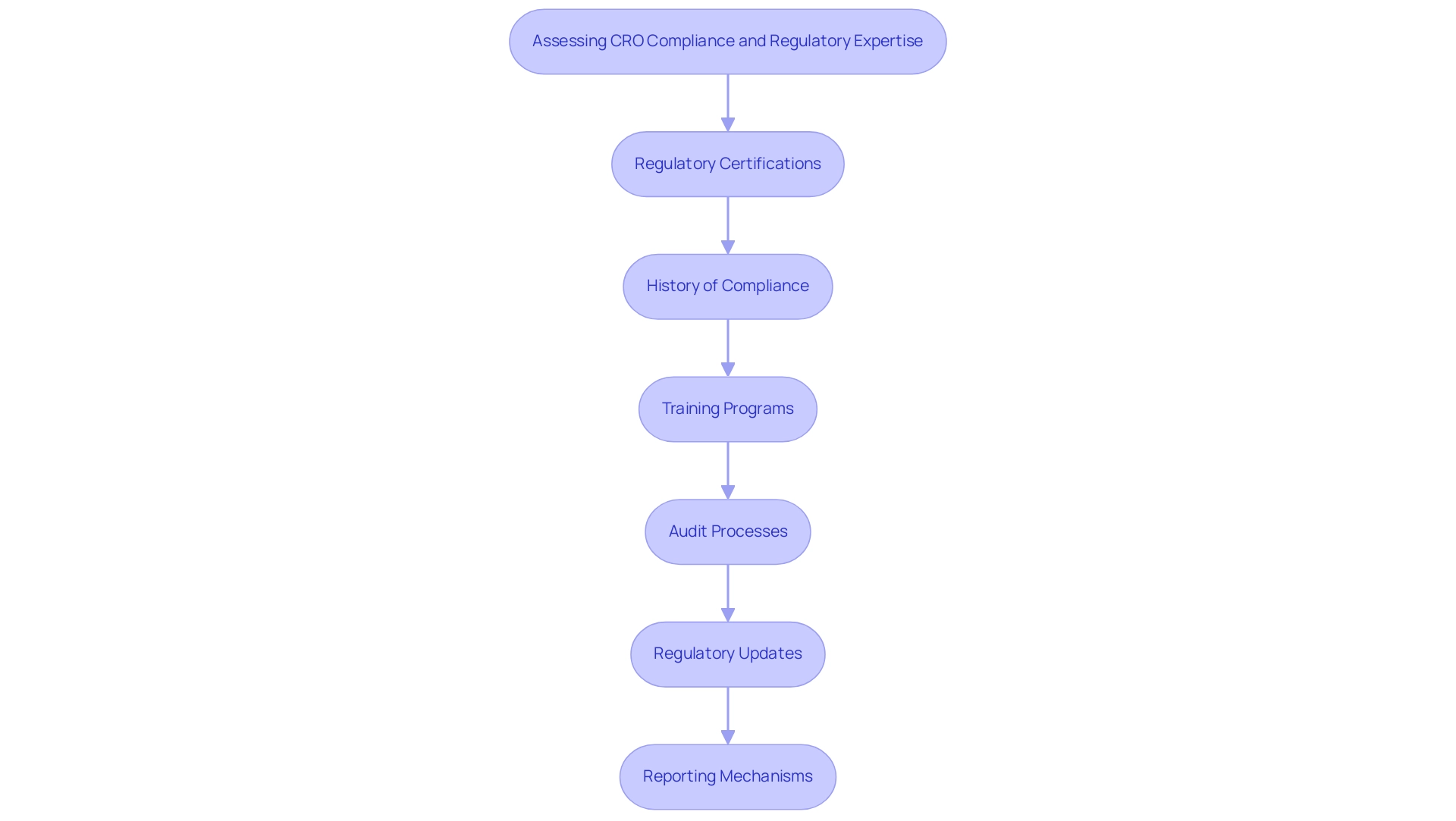
When assessing a CRO's compliance and regulatory expertise, consider the following:
- Regulatory Certifications: Verify the CRO's certifications and accreditations relevant to research, such as ISO certifications or Good Clinical Practice (GCP) compliance.
- History of Compliance: Investigate the CRO's history regarding compliance with regulatory bodies. A strong track record suggests dependability and compliance with guidelines, especially in the context of the comprehensive services they provide, including feasibility studies, setup for experiments, and project management.
- Training Programs: Inquire about the training programs the CRO has in place for staff regarding regulatory compliance and ethical conduct in research. This is vital for ensuring that all personnel are equipped to handle the complexities of clinical research.
- Audit Processes: Understand the CRO's internal audit processes for ensuring compliance. Regular audits can help identify and rectify potential issues before they escalate, reinforcing the integrity of the testing process.
- Regulatory Updates: Assess how the CRO stays updated with changing regulations. Continuous education and adaptation to new guidelines are essential for maintaining compliance. This is particularly crucial for navigating the regulatory environment in Colombia, where knowledge like that of Katherine Ruiz, a Regulatory Affairs specialist at a Medical Device CRO Colombia, can significantly improve the approval process.
- Reporting Mechanisms: Ensure the CRO has robust reporting systems in place for study status, inventory, and adverse events, both serious and non-serious, to maintain transparency and accountability throughout the research.
By evaluating these factors, stakeholders can ensure they choose a CRO that prioritizes compliance, thereby safeguarding the integrity of their studies. Moreover, grasping the procedure for acquiring research study approval in Colombia—including IRB/EC authorization, INVIMA approval, and MinCIT import permits—can further improve the effectiveness of the services offered by the Medical Device CRO Colombia.
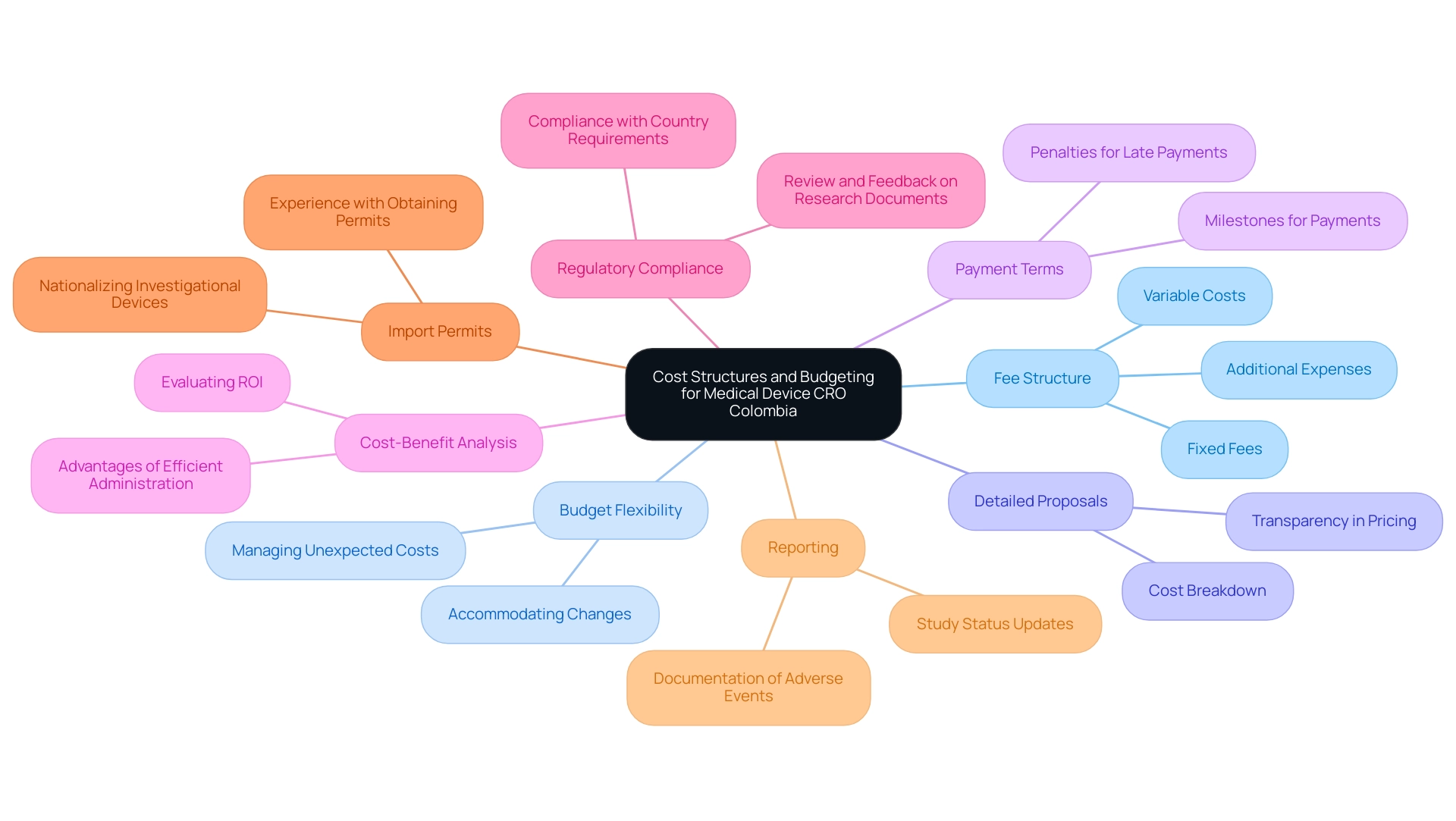
Understanding Cost Structures and Budgeting
When understanding the cost structures and budgeting for a Medical Device CRO Colombia, consider these aspects:
- Fee Structure: Inquire about the CRO’s fee structure, including fixed fees, variable costs, and any additional expenses that may arise during the trial.
- Budget Flexibility: Assess the CRO's flexibility in accommodating budget changes or unexpected costs.
A Medical Device CRO Colombia that can adjust to financial changes can be a valuable ally, particularly in dynamic settings where study parameters may evolve.
-
Detailed Proposals: Request detailed proposals that outline all costs associated with the project. Transparency in pricing is essential to prevent budget overruns and ensure effective resource allocation.
-
Payment Terms: Understand the payment terms and conditions, including milestones for payments and any penalties for late payments.
-
Cost-Benefit Analysis: Conduct a cost-benefit analysis to evaluate the potential return on investment (ROI) based on the CRO's services and expertise. Efficient administration of clinical evaluations by a Medical Device CRO Colombia, which includes feasibility assessments, site selection, compliance reviews, and reporting, can lead to considerable advantages such as job creation and healthcare enhancement in local economies.
-
Regulatory Compliance: Ensure the Medical Device CRO Colombia provides thorough review and feedback on research documents to comply with country requirements, as this is crucial for the project's success.
-
Import Permits: Consider the experience of the Medical Device CRO Colombia with obtaining import permits and nationalizing investigational devices, which are essential for conducting studies in different regions.
-
Reporting: Evaluate the CRO's approach to reporting, including study status updates and documentation of serious and non-serious adverse events, to maintain transparency and compliance throughout the trial process.
By thoroughly understanding these financial considerations and the broader impact of CRO services, stakeholders can make informed decisions about budgeting and selecting a CRO that aligns with their financial capabilities and project needs.
Conclusion
The selection of a Contract Research Organization (CRO) is a critical decision for Medtech startups aiming to navigate the complexities of clinical trials, particularly in the dynamic landscape of Latin America. The insights provided throughout this article underscore the importance of evaluating key criteria such as:
- Expertise
- Regulatory knowledge
- Project management capabilities
- Compliance practices
Each of these factors plays a vital role in ensuring that clinical trials not only meet regulatory standards but also achieve successful outcomes.
Moreover, understanding the unique challenges faced in the Latin American market—such as regulatory hurdles and communication barriers—highlights the necessity for strategic partnerships with CROs that have proven experience in the region. Organizations like bioaccess® exemplify how effective collaboration can lead to accelerated Medtech innovations, as evidenced by successful case studies in clinical research.
Ultimately, stakeholders must approach the selection of a CRO with a comprehensive understanding of their specific project needs. By thoroughly assessing the capabilities and track records of potential partners, organizations can enhance their chances of conducting successful clinical trials that contribute to the advancement of medical technologies. This strategic approach not only fosters successful outcomes but also paves the way for innovative solutions in healthcare, benefiting patients and the industry alike.




