Introduction
The landscape of medical device clinical trials in Latin America is rapidly evolving, presenting unique opportunities and challenges for stakeholders in the industry. With a diverse patient population and a growing pool of local expertise, countries like Paraguay are positioning themselves as key players in this field. Understanding the intricacies of the region's healthcare infrastructure, regulatory environment, and cultural nuances is essential for successful trial management.
As organizations seek to navigate this complex terrain, the role of Contract Research Organizations (CROs) becomes increasingly critical. Their expertise not only streamlines the clinical trial process but also ensures compliance with local regulations, ultimately enhancing the likelihood of successful outcomes.
This article delves into the various dimensions of conducting medtech clinical trials in Latin America, offering insights into:
- Regulatory requirements
- The selection of qualified CROs
- The importance of robust project management practices
Understanding the Landscape of Medtech Clinical Trials in Latin America
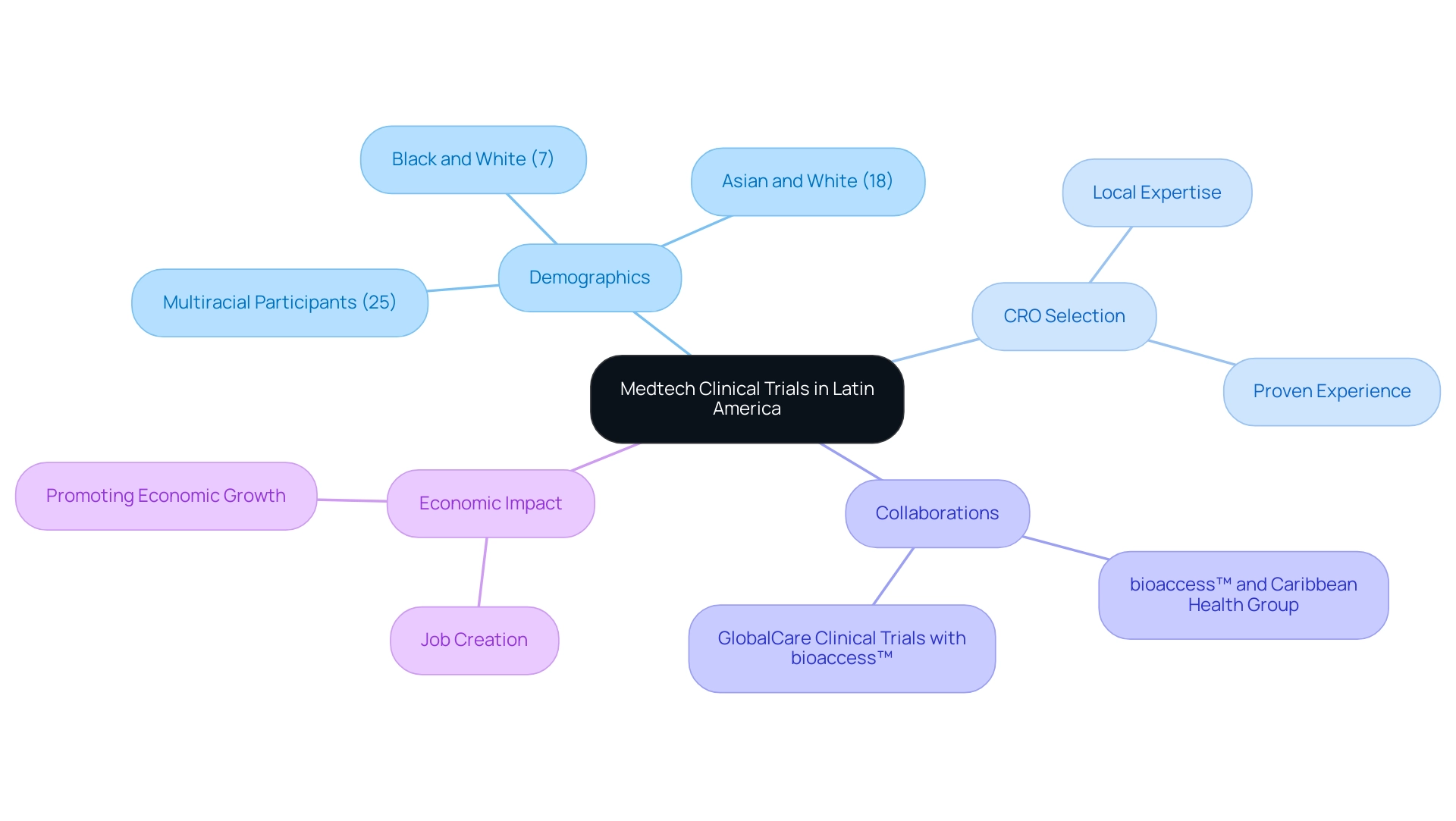
Latin America has emerged as a crucial area for device research, driven by its diverse patient demographics—140 participants (25%) self-reported as multiracial, including 100 (18%) identifying as Asian and White and 40 (7%) as Black and White. This diversity highlights the importance of understanding the patient population in research trials, particularly considering Annette Flanagin's assertion that 'Neglecting to report race and ethnicity in health and medical research disregards the reality of social stratification, injustices, and inequities.' Nations like Paraguay are increasingly acknowledged for their strategic benefits in the realm of Medical Device CRO Paraguay, bolstered by a pool of local expertise and a rising number of trained professionals skilled in research methodologies.
A thorough understanding of the region's unique characteristics—including cultural nuances and the intricacies of its healthcare infrastructure—is essential for selecting a competent Contract Research Organization (CRO). Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as the most appealing location for research in Latin America, with the clear backing of Colombia's Minister of Health. Moreover, collaborations such as that of GlobalCare Clinical Trials with bioaccess™ improve ambulatory services in Colombia, achieving over a 50% reduction in recruitment duration and a retention rate surpassing 95%.
Potential clients should prioritize CROss with proven experience in managing studies specifically within the Latin American context, as this expertise can significantly enhance the likelihood of project success. The guidance for reporting race and ethnicity in research articles provided by the JAM Network journals serves as a concrete example of best practices in demographic reporting, ensuring that findings can be appropriately contextualized and understood. With the expected growth in research trials in Paraguay by 2024, stakeholders must stay informed about the evolving landscape of the Medical Device CRO Paraguay to fully leverage the potential of this promising market.
Additionally, the impact of Medtech research studies on local economies is significant, fostering job creation and promoting economic growth, which are essential considerations for stakeholders in the region.
Navigating Regulatory Requirements for Medical Devices in Paraguay
In Paraguay, the regulatory framework for health devices is overseen by the National Directorate of Health. The healthcare device registration process involves several key steps:
- Preparation and submission of a comprehensive application dossier, which includes:
- Technical documentation
- Clinical trial data
- Evidence of compliance with local standards
This process can be challenging for device startups, given the regulatory hurdles, competition, and recruitment issues they face.
After submission, the application undergoes a detailed review by regulatory authorities, which may include inspections of manufacturing facilities. Once approved, the device is granted market authorization. Choosing a Medical Device CRO Paraguay, like bioaccess®, which specializes in navigating these regulatory requirements, is vital, as non-compliance can lead to significant delays and increased costs.
A reputable CRO will have established connections with regulatory authorities, facilitating smoother interactions and expediting the approval process. Furthermore, it is essential for these organizations to remain informed about any changes in the regulatory landscape, ensuring they can adapt their strategies and maintain compliance effectively. Bioaccess® offers tailored solutions as a Medical Device CRO Paraguay, which include:
- Comprehensive trial setup
- Import permits
- Project management
- Reporting services to address the unique challenges faced by startups.
Katherine Ruiz, an expert in Regulatory Affairs for healthcare devices and in vitro diagnostics in Colombia, exemplifies the caliber of professionals who can help navigate these complexities, ensuring a streamlined path to market for innovative technologies. To learn more about how bioaccess® can assist your device initiatives, consider booking a consultation.

Assessing the Experience and Expertise of Potential CROs
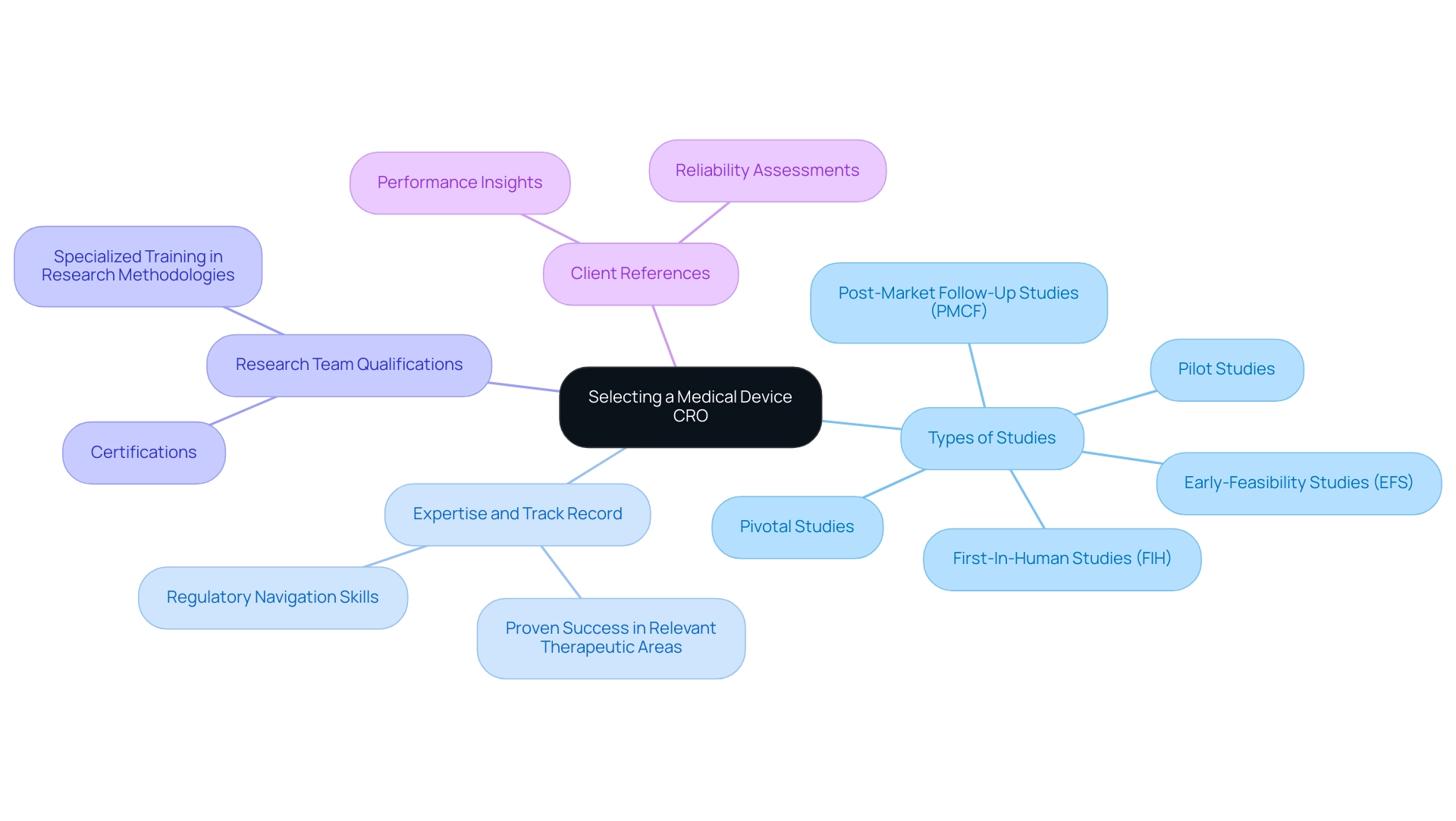
When selecting a Medical Device CRO Paraguay for device studies, it is essential to evaluate their expertise with investigations that closely align with your specific project. bioaccess®, with over 20 years of expertise in Medtech, serves as a Medical Device CRO Paraguay, specializing in managing a variety of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF) in Latin America.
This thorough assessment should include a review of their history of successful experiments, particularly in therapeutic areas relevant to your device.
A strong indicator of a Medical Device CRO Paraguay's capability is their proven track record; for instance, bioaccess® is the only CRO vetted and approved by the U.S. Commercial Service to assist U.S. medical device companies in Colombia, showcasing their adeptness in navigating regulatory landscapes. Their ability to provide comprehensive trial management services—encompassing feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—ensures a streamlined process.
Furthermore, bioaccess®’s customized study design and protocol development are essential for balancing stakeholder interests in pivotal trials, ensuring that studies meet the needs of all parties involved. The qualifications of the CRO's research team should be examined, focusing on their certifications and specialized training in research methodologies. Client references play a vital role in this evaluation process, offering valuable insights into the CRO's performance and reliability.
As Liz Galle, former VP of Global Clinical Research, emphasizes, 'As a startup organization, CVRx had to secure the right partnerships to obtain safety and effectiveness evidence required for FDA approval.' We were fortunate to choose bioaccess® as our preferred research partner – they were with us throughout the journey, continually providing their extensive statistical expertise and development support. This partnership not only facilitated CVRx's successful navigation of the FDA approval process but also led to their growth into a publicly traded company, bringing life-saving technology to heart failure patients throughout the US. This highlights the significance of selecting a Medical Device CRO Paraguay with a strong reputation, such as bioaccess®, which has a proven track record of success in the medical device industry, enabling them to skillfully handle the intricacies inherent in research.
Evaluating the CRO's Project Management Capabilities
A thorough evaluation of management methodologies in Contract Research Organizations (CROs) is essential for determining their effectiveness in handling multiple initiatives and timelines. A proficient CRO should showcase strong strategies for risk management and problem resolution, which are essential in managing the intricacies of clinical studies. Our service capabilities encompass comprehensive offerings such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Testing setup
- Import permits
- Project management and monitoring
- Detailed reporting on study status and adverse events
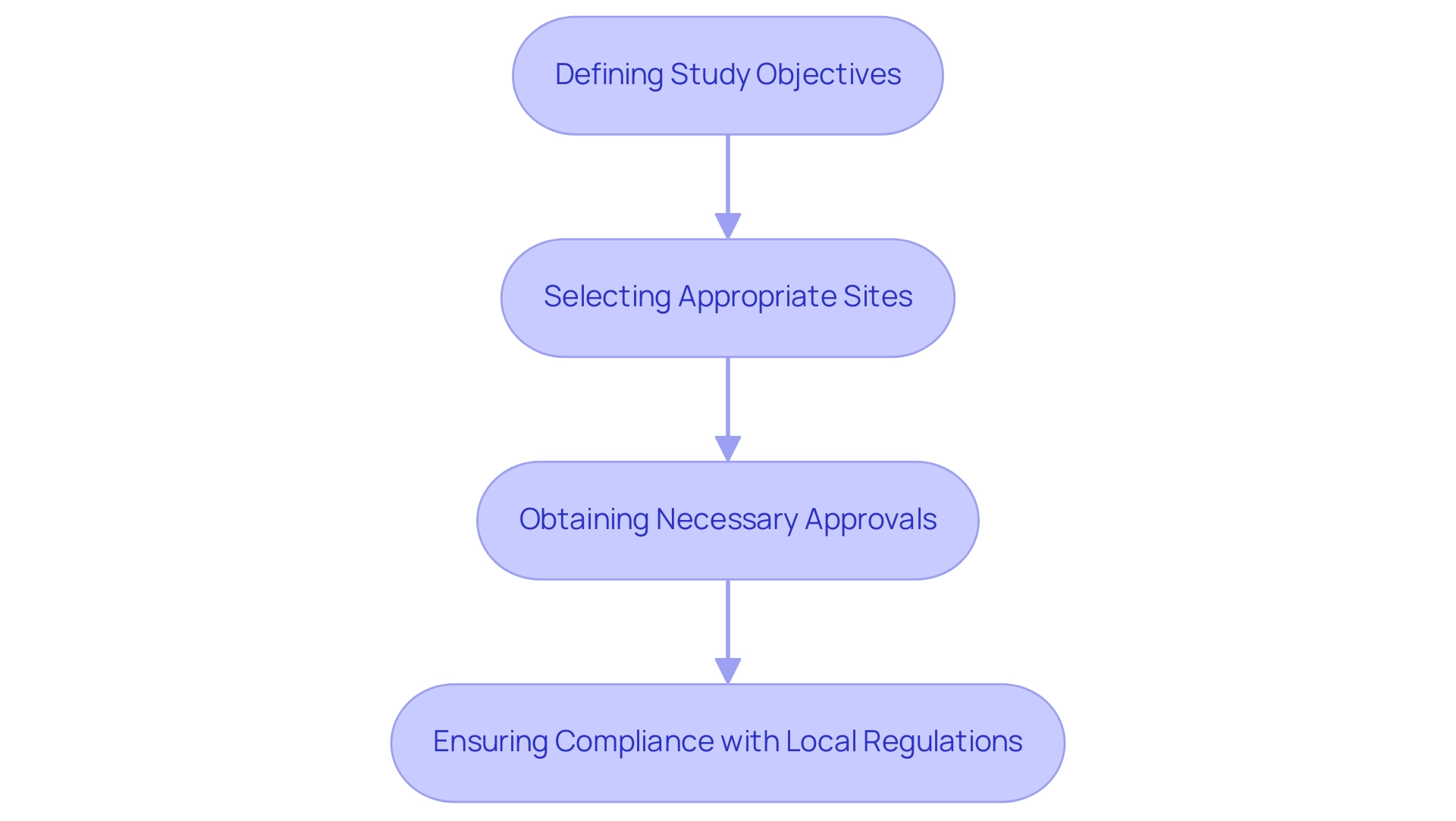
The setup process typically involves several key steps:
- Defining study objectives
- Selecting appropriate sites
- Obtaining necessary approvals
- Ensuring compliance with local regulations
Clear communication channels and regular reporting mechanisms are vital for keeping stakeholders informed throughout the trial process. As emphasized by Novotech, a worldwide frontrunner in research, their dedication to communication is clear in their assistance for more than 5,000 research initiatives across 34 locations with a devoted team of over 3,000 experts.
Notably, Novotech has played an instrumental role in advancing precision oncology since its founding in 1997, showcasing their expertise through multiple award-winning clinical initiatives. Their contributions have significantly influenced the evolution of cancer treatment, underscoring the importance of effective management methodologies. By promoting international collaboration and the exchange of knowledge, CROs can drive innovation in Medtech, ultimately leading to better global health outcomes.
Furthermore, the compliance review process ensures that all study documents meet the specific requirements of the countries involved, which is crucial for regulatory approval and ethical considerations. The adoption of advanced management tools and technologies is crucial for enhancing operational efficiency and transparency. Readers are encouraged to explore Novotech's whitepaper for deeper insights into precision medicine, cancer research, and novel therapies.
By implementing these best practices, a CRO can significantly improve its project management capabilities, ultimately leading to more successful outcomes in clinical trials.
Conclusion
The evolution of medical device clinical trials in Latin America presents both significant opportunities and challenges for stakeholders. A comprehensive understanding of the region's diverse patient demographics and healthcare infrastructure is paramount for successful trial execution. The increasing recognition of countries like Paraguay, bolstered by local expertise and strategic collaborations, exemplifies the region's potential as a hub for clinical research.
Navigating the regulatory landscape is a critical component in the success of these trials. The intricate process of medical device registration necessitates the involvement of experienced Contract Research Organizations (CROs) to ensure compliance and efficiency. By selecting a CRO with proven capabilities, such as bioaccess®, stakeholders can mitigate risks associated with regulatory hurdles and streamline the path to market.
Furthermore, the importance of robust project management practices cannot be overstated. A CRO that demonstrates effective project management methodologies can significantly enhance the likelihood of trial success. This involves not only meticulous planning and execution but also clear communication and a commitment to adapting to the evolving regulatory environment.
In summary, stakeholders in the medtech industry must remain vigilant and informed as the landscape of clinical trials in Latin America continues to evolve. By leveraging local expertise, adhering to regulatory requirements, and selecting the right CRO, organizations can position themselves for success in this dynamic market. The potential for growth and economic impact in the region is substantial, making it imperative for all involved to embrace these opportunities thoughtfully and strategically.
Frequently Asked Questions
Why is Latin America important for device research?
Latin America has a diverse patient demographic, which is crucial for understanding various health conditions in research trials. This diversity includes significant multiracial representation, which emphasizes the need to consider race and ethnicity in health research.
What is the significance of understanding patient demographics in research trials?
Understanding patient demographics is essential as it addresses social stratification, injustices, and inequities in health and medical research, ensuring findings are relevant and contextualized.
What role do Contract Research Organizations (CROs) play in device studies in Latin America?
CROs are vital for navigating the regulatory landscape and managing studies. They help ensure compliance with local regulations and provide expertise in research methodologies, which enhances project success.
What are some challenges faced by device startups in Paraguay?
Device startups in Paraguay encounter regulatory hurdles, competition, and recruitment issues, making it crucial to choose a knowledgeable CRO to help navigate these complexities.
What steps are involved in the healthcare device registration process in Paraguay?
The registration process includes preparing and submitting a comprehensive application dossier, which contains technical documentation, clinical trial data, and evidence of compliance with local standards. This is followed by a detailed review by regulatory authorities.
How does bioaccess® assist in the regulatory process for medical devices in Paraguay?
Bioaccess® specializes in navigating regulatory requirements, offering services such as comprehensive trial setup, import permits, project management, and reporting, which are essential for startups facing regulatory challenges.
What types of studies does bioaccess® manage as a Medical Device CRO in Paraguay?
Bioaccess® manages various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
How can clients evaluate the capabilities of a Medical Device CRO?
Clients should review the CRO's history of successful experiments in relevant therapeutic areas, assess the qualifications of the research team, and consider client references to gauge performance and reliability.
What impact do Medtech research studies have on local economies in Latin America?
Medtech research studies foster job creation and promote economic growth, making them significant for stakeholders in the region.
Why is effective project management important for CROs?
Effective project management ensures that clinical studies are handled efficiently, with strong strategies for risk management and problem resolution, which are crucial for successful outcomes.
What best practices should CROs adopt to improve project management capabilities?
CROs should implement clear communication channels, regular reporting mechanisms, and advanced management tools to enhance operational efficiency and transparency in clinical trials.

