Overview
Contract Research Organizations (CROs) play a crucial role in clinical trials by managing operations, ensuring regulatory compliance, and facilitating patient recruitment, which ultimately enhances the efficiency and effectiveness of medical research. The article emphasizes that CROs not only streamline the study process through their comprehensive services but also foster strategic partnerships with sponsors, leveraging technology and collaboration to address challenges and improve outcomes in clinical research.
Introduction
In the realm of clinical research, Contract Research Organizations (CROs) have emerged as indispensable allies for pharmaceutical, biotechnology, and medical device companies. Their expertise spans the entire clinical trial continuum, from managing operations and executing patient recruitment strategies to ensuring compliance with rigorous regulatory standards.
As the prevalence of chronic diseases continues to rise, particularly in regions like Europe and Latin America, the role of CROs becomes increasingly critical in driving innovative treatments and improving patient outcomes.
This article delves into the multifaceted services offered by CROs, the evolving dynamics between sponsors and these organizations, and the future trends that promise to reshape the landscape of clinical trials through technological advancements and a commitment to diversity.
By understanding the essential contributions of CROs, stakeholders can better navigate the complexities of clinical research and enhance the efficiency and effectiveness of their trials.
Defining Contract Research Organizations: Their Essential Role in Clinical Trials
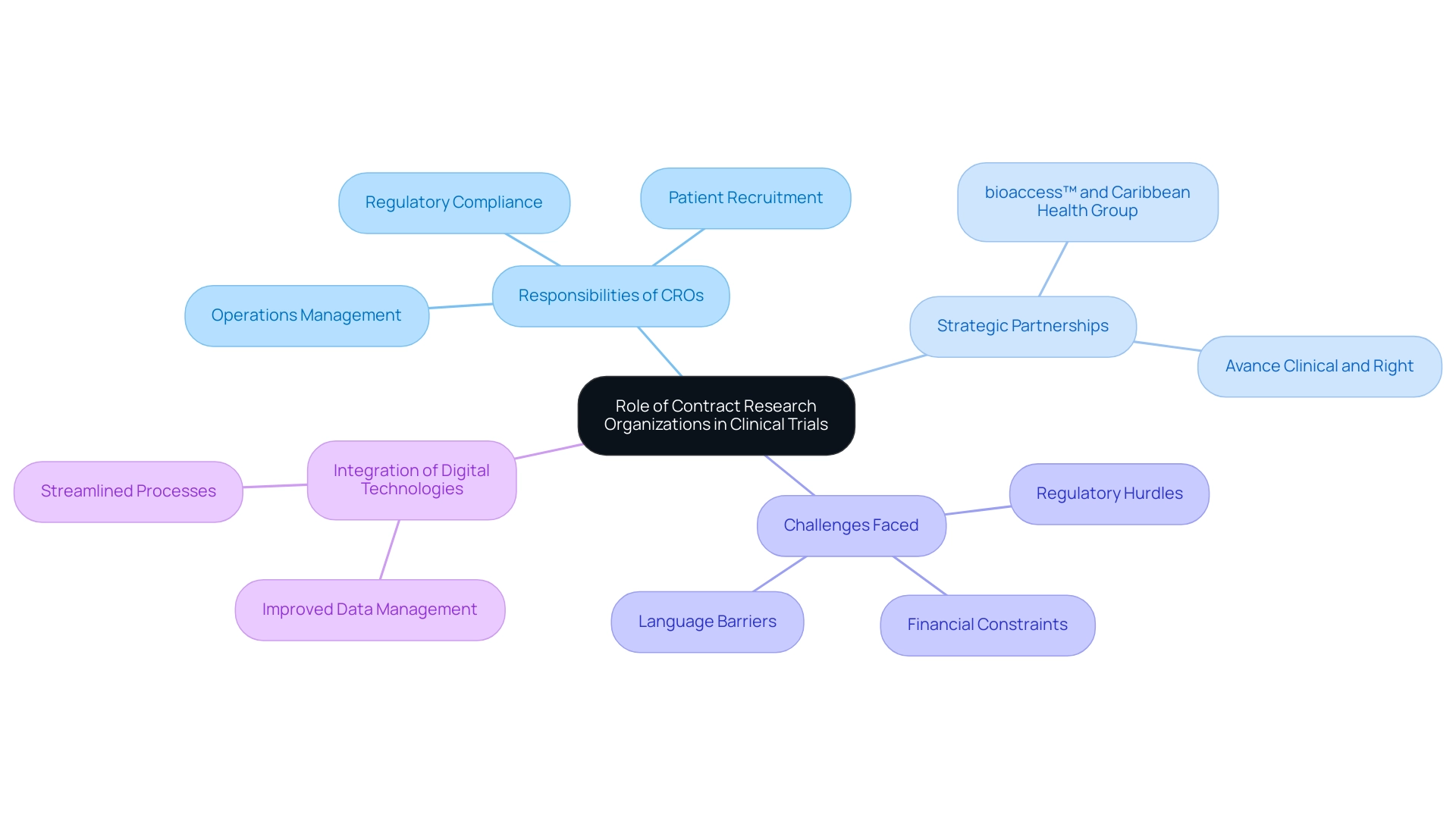
Contract Research Organizations (CROs) serve as specialized collaborators in the pharmaceutical, biotechnology, and medical device industries, offering extensive assistance throughout Contract Research Clinical Trials. Their responsibilities include managing operations, executing patient recruitment strategies, and ensuring compliance with regulatory standards. This multifaceted position is crucial for the effective management of research studies, particularly considering the staggering fact that nearly 60 million individuals in Europe are impacted by multiple chronic illnesses.
In Latin America, the collaboration between bioaccess™ and Caribbean Health Group exemplifies how strategic partnerships can enhance clinical research, with the support of Colombia's Minister of Health positioning Barranquilla as a leading destination for studies. By partnering with CROs for Contract Research Clinical Trials, sponsors can access specialized expertise, significantly reduce operational costs, and enhance study timelines. This is especially vital in tackling the intricacies of chronic disease management, where successful studies can result in advancements in treatment.
However, US Medtech companies also face significant challenges such as regulatory hurdles, financial constraints, and language barriers, which can complicate their operations in Latin America. The recent collaboration between Avance Clinical and Right demonstrates this trend; by incorporating innovative GenAI technologies into research networks, they aim to improve cooperation and operational efficiency within the research ecosystem. These digital technologies can streamline processes, improve data management, and facilitate communication, thereby addressing some of the key challenges faced by Medtech companies.
Notably, the integration of digital technologies presents a substantial opportunity for contract research clinical trials to bolster collaboration and operational efficiency in this complex landscape. As pointed out by Heidi Hennigan, a Senior Principal Biostatistician at a prominent international pharmaceutical company, the sector's increasing dependence on specialized positions highlights the crucial approach of subcontracting research management to Contract Research Clinical Trials.
Comprehensive Services Offered by CROs in Clinical Trial Management
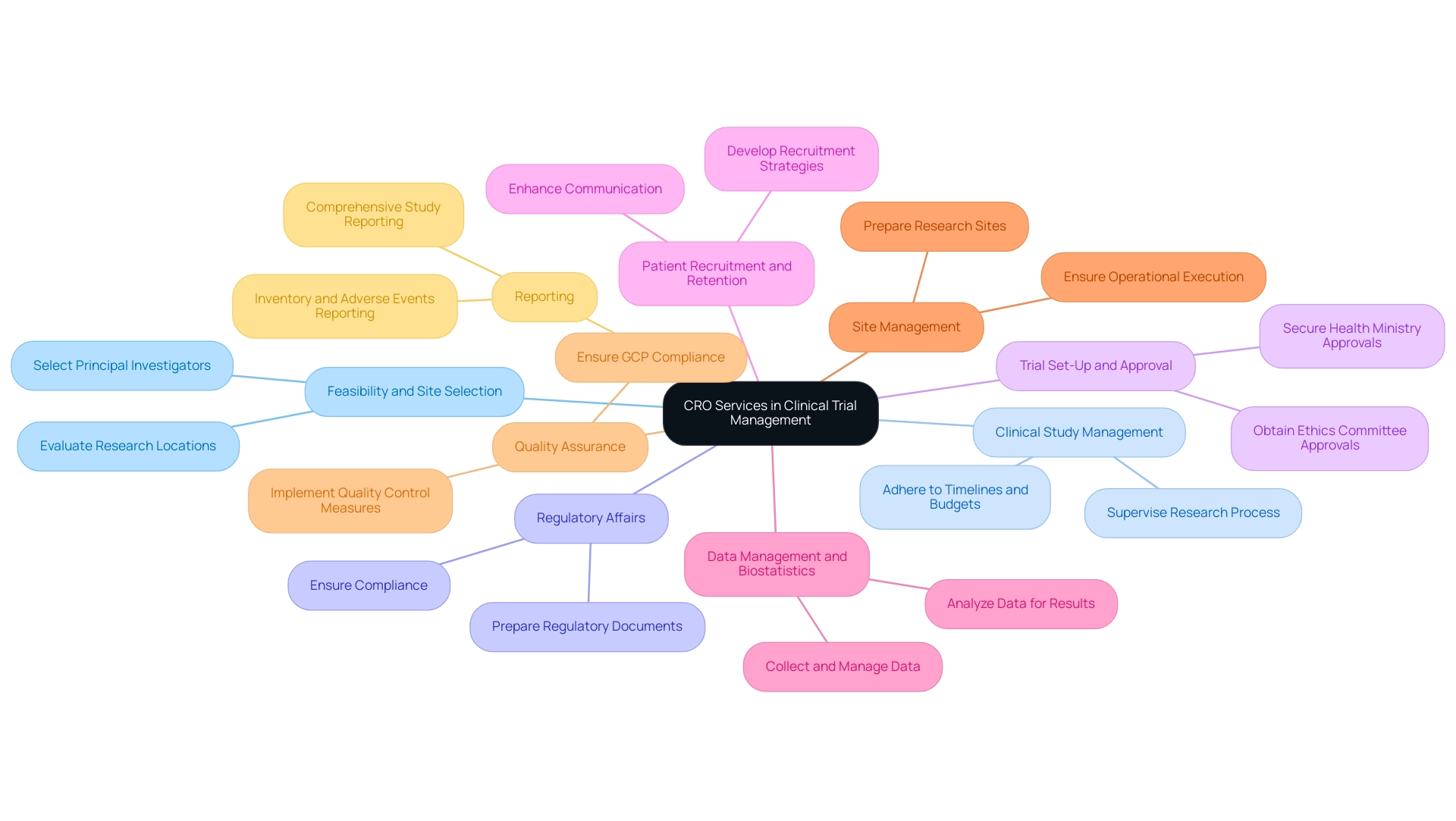
Contract Research Organizations offer a comprehensive suite of services that are indispensable for the successful management of Contract Research Clinical Trials, particularly for first-in-human medical devices in Colombia. Outsourcing to a CRO can streamline the study process for Contract Research Clinical Trials and seamlessly integrate various components, enhancing overall efficiency. These services include:
- Feasibility and Selection of Research Site and Principal Investigator (PI): Contract Research Organizations evaluate potential research locations and choose suitable principal investigators, ensuring the right fit for the study's needs.
- Clinical Study Management: CROs supervise the complete research process, guaranteeing that all stages from planning to execution are conducted efficiently, adhering rigorously to timelines and budgets.
- Regulatory Affairs: They play a critical role in preparing and submitting regulatory documents, ensuring compliance with local and international regulations. Evaluating a CRO's record of regulatory adherence is essential, particularly in understanding the regulatory environment in Colombia, which is recognized for its speed and efficiency in facilitating Contract Research Clinical Trials.
- Trial Set-Up, Start-Up, and Approval: This includes obtaining necessary approvals from ethics committees and health ministries, which is crucial for initiating trials.
- Patient Recruitment and Retention: Developing effective strategies for recruiting and retaining participants is essential for trial success. Effective communication, which is the cornerstone of successful clinical research, greatly enhances collaboration and compliance throughout this process.
- Data Management and Biostatistics: Contract Research Organizations excel in collecting, managing, and analyzing data, which is crucial for providing accurate and timely results. Leading contract research organizations utilize advanced technologies and robust data management systems to streamline data collection, cleaning, validation, and storage. This guarantees the integrity of data gathered during tests, allowing informed choices and backing evidence-based studies for new medications and interventions.
- Site Management: Collaborating with research sites to guarantee they are well-prepared and equipped for the study is a key responsibility of contract research organizations, ensuring smooth operational execution.
- Quality Assurance: Implementing rigorous quality control measures is fundamental to upholding data integrity and ensuring compliance with Good Clinical Practice (GCP) standards.
- Reporting: Comprehensive reporting on study status, inventory, and serious and non-serious adverse events is critical for maintaining transparency and regulatory compliance.
As the clinical research landscape evolves, Contract Research Clinical Trials services remain crucial in enhancing the efficiency and effectiveness of clinical studies, ultimately contributing to the successful introduction of new medical interventions in Colombia. To learn more about how we can assist you, BOOK A MEETING.
The Evolving Relationship Between CROs and Sponsors: Building Strategic Partnerships
The relationship between sponsors and Contract Research Organizations in Contract Research Clinical Trials has experienced a notable change, shifting from a purely transactional dynamic to one focused on strategic partnerships. This change indicates an increasing awareness among sponsors of the value that contract research clinical trials can provide beyond operational assistance, particularly in enhancing study efficiency through insights and innovations. Several key factors are influencing this evolution:
-
Collaboration: A collaborative approach is increasingly vital, as it fosters open communication and effective problem-solving.
As demonstrated by Dr. John B. Simpson's experiences with Avinger's OCT-guided atherectomy studies in Cali, Colombia, cooperation with experts in Contract Research Clinical Trials from LATAM has been vital for addressing challenges and ensuring flexible responses during evaluations.
-
Trust: Building trust between Contract Research Organizations and sponsors is crucial for aligning objectives and maintaining transparency throughout the evaluation process. Trust serves as a foundation for a fruitful relationship, allowing both parties to strive for common goals without the obstacle of miscommunication or misalignment.
-
Shared Objectives: Establishing shared objectives enables both executives and sponsors to focus on successful outcomes. This integrated method not only simplifies study management but also improves the overall efficiency of the medical investigation process.
For example, Medpace's ambitious plans to add 1,500 positions and invest $150 million in expanding its operations over the next six years demonstrate how Contract Research Clinical Trials organizations are positioning themselves to meet the rising demands for partnership. Moreover, the extensive service capabilities of contract research organizations, particularly in the realm of Contract Research Clinical Trials, including feasibility and selection of study sites, principal investigator (PI) selection, compliance document reviews, study setup and approval processes (including ethics committee and health ministry), import permits for investigational devices, project management, and detailed reporting on study status and adverse events, emphasize their essential role in the success of medical device evaluations.
Furthermore, the influence of medtech studies goes beyond individual assessments, aiding local economies through job creation, economic growth, healthcare enhancement, and international collaboration. By offering vital services, contract research organizations that conduct Contract Research Clinical Trials not only enable successful studies but also assist in boosting local employment opportunities and improving healthcare access. Ergomed's 25 years of experience in the industry exemplifies the benefits of fostering strategic partnerships, operating in over 100 countries and serving more than 300 clients while maintaining a small company feel.
This approach reflects the trust and shared objectives essential for success.
In addition, Proclinical is currently recruiting for a Senior Principal Biostatistician for a permanent remote position within the United Kingdom, showcasing the ongoing demand for skilled professionals in the CRO landscape. As the environment of medical studies continues to change, the significance of strong collaborations between sponsors and contract research organizations in contract research clinical trials will only grow, ultimately affecting success rates and the overall efficiency of medical evaluations.
Enhancing Clinical Trial Efficiency and Compliance Through CROs
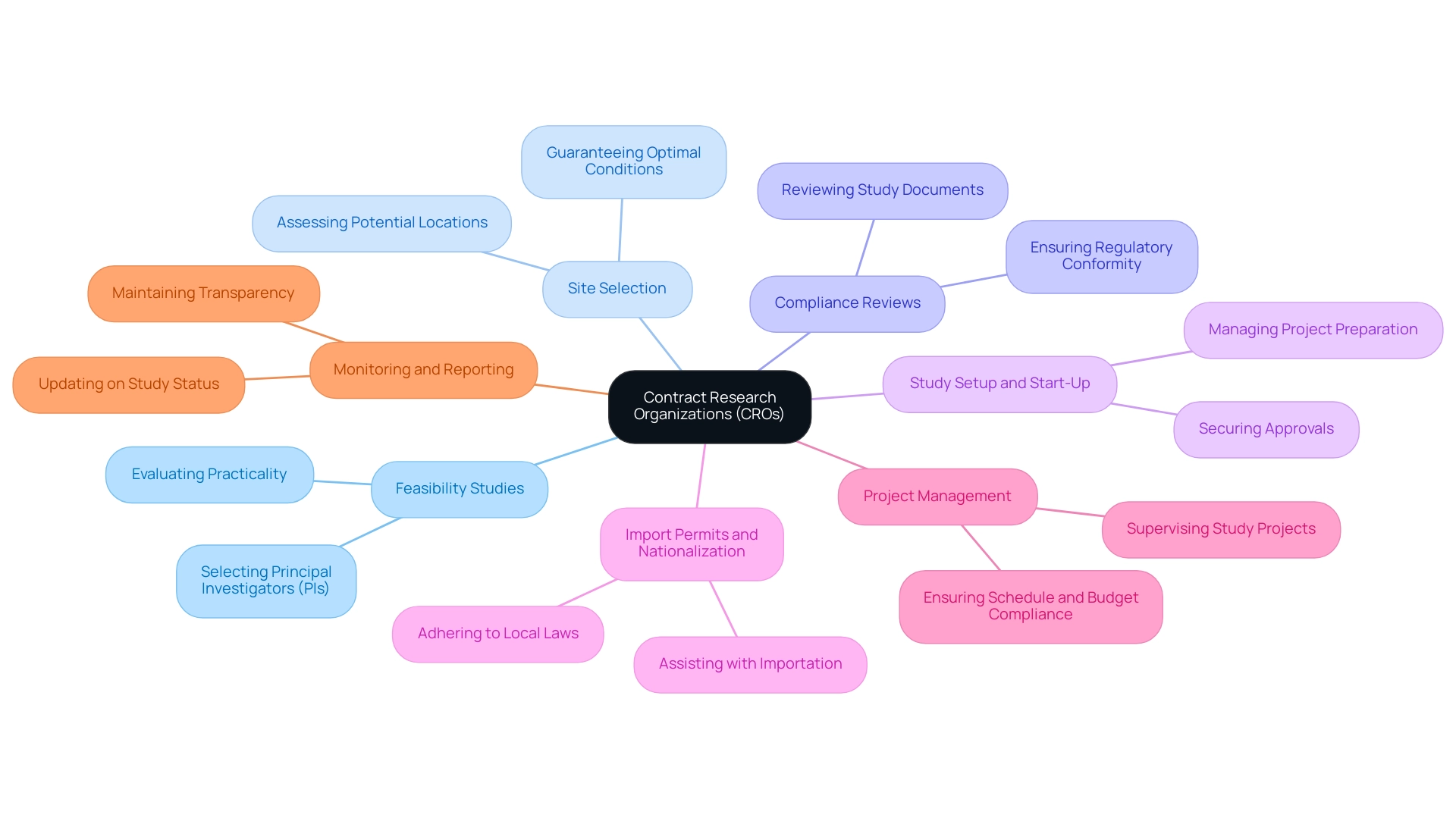
Contract Research Organizations are vital to improving the effectiveness of medical studies through their extensive abilities, which encompass:
- Feasibility Studies: Evaluating the practicality of conducting research in specific locations and selecting appropriate principal investigators (PIs).
- Site Selection: Recognizing and assessing potential research locations to guarantee optimal conditions for the study.
- Compliance Reviews: Offering review and feedback on study documents to ensure conformity to local and international regulations, which is essential for avoiding costly setbacks.
- Study Setup and Start-Up: Managing the preparation and initiation of research projects, including securing necessary approvals from ethics committees and health ministries.
- Import Permits and Nationalization: Assisting with the importation and nationalization of investigational devices to adhere to local laws.
- Project Management: Supervising the entire study project, ensuring it stays on schedule and within budget.
- Monitoring and Reporting: Frequently updating on study status, inventory, and serious and non-serious adverse events to maintain transparency and compliance.
These organizations take a proactive approach in identifying potential risks and developing effective mitigation strategies for Contract Research Clinical Trials, ensuring studies remain compliant and on schedule. By providing comprehensive training for site personnel and investigators, contract research organizations ensure all parties involved are well-versed in regulatory requirements and study protocols. This training boosts overall experiment efficiency, demonstrated by case studies that show the application of automated machine learning algorithms for statistical tests, conserving programming time and enhancing adaptability in study designs.
In summary, contract research organizations not only optimize research processes but also support local economies through job creation, economic growth, and better healthcare outcomes by conducting contract research clinical trials, thus advancing global health enhancement through international collaboration and innovation in Medtech.
Future Trends in Contract Research Organizations: Innovation and Diversity in Clinical Trials
The future landscape of Contract Research Organizations (CROs) is on the verge of significant change, driven by technological advancements and a commitment to enhancing diversity in Contract Research Clinical Trials. Several key trends are emerging:
-
Comprehensive Clinical Trial Management Services: Our service capabilities encompass feasibility studies, selection of research sites and principal investigators, and thorough compliance reviews of study documents. We assist in establishing the setup, including ethics committee and health ministry approvals, import permits, and nationalization of investigational devices.
Effective project management and detailed reporting on study status, inventory, and adverse events are critical to our approach.
-
Technology Integration: The adoption of digital tools, including electronic data capture (EDC) and artificial intelligence (AI), is revolutionizing data management and analysis. Machine learning, in particular, is increasingly applied in imaging and diagnosis within healthcare, further enhancing the capabilities of CROs. As the process of composing and approving study protocols can take anywhere from 5 to 20 weeks, these technologies promise to streamline workflows and enhance efficiency.
For instance, decentralized studies (DCTs) are estimated to reduce study expenses by 10-25% due to fewer locations and lower patient visit costs, enhancing patient engagement through 'bring your own device' (BYOD) capabilities.
-
Participant-Focused Approaches: A notable shift towards participant-focused design is prioritizing the needs and experiences of individuals. This focus is expected to significantly improve recruitment and retention rates, ultimately resulting in more effective and relevant outcomes. For example, recent surveys indicate that 94% of patients are likely to use mobile applications for research studies, emphasizing the convenience and engagement potential of these methods.
-
Diversity in Studies: There is a growing focus on ensuring diversity within research populations. This initiative is essential for producing findings that are applicable across a broader demographic spectrum, thereby enhancing patient outcomes. As pointed out by Mike Cioffi, Senior Vice President of Clinical Solutions and Strategic Partnerships at WCG, “While there are certainly limitations to current technologies and biomarker identification, and despite precision medicine being further researched, this multidisciplinary approach will redefine our ability to improve the treatment and management of epilepsy in the future.” This viewpoint emphasizes the necessity for inclusivity in research populations to ensure that advancements in treatment benefit all demographics.
-
Collaborative Ecosystems: CROs are increasingly operating within collaborative ecosystems that include sponsors, regulatory bodies, and patient advocacy groups. This partnership is vital for improving the quality and compliance of research studies, particularly considering new regulatory guidance like ICH E8 and the FDA’s Diversity guidance, which emphasizes the significance of quality in study design and implementation. Additionally, Medtech firms in Latin America encounter particular obstacles such as regulatory hurdles, language barriers, and fragmented resources, which impede effective communication and collaboration with American research clients. Addressing these challenges through collaborative efforts is essential for improving the overall landscape of Contract Research Clinical Trials in the region.
These trends collectively signal a significant shift in how Contract Research Clinical Trials will be approached by CROs, embracing technology and inclusivity to foster innovation and improve patient care.
Conclusion
The role of Contract Research Organizations (CROs) in clinical trials is increasingly vital as the healthcare landscape evolves. By providing comprehensive services ranging from feasibility studies to regulatory compliance, CROs significantly enhance the efficiency and effectiveness of clinical trials. Their expertise not only aids pharmaceutical, biotechnology, and medical device companies in navigating the complexities of research but also addresses the pressing needs arising from the growing prevalence of chronic diseases across regions such as Europe and Latin America.
The evolving relationship between CROs and sponsors reflects a shift towards strategic partnerships, emphasizing collaboration, trust, and shared objectives. This transformation is crucial for overcoming the challenges inherent in clinical research, ultimately leading to successful trial outcomes. Moreover, with the integration of advanced technologies and a focus on patient-centric approaches, CROs are poised to drive innovation and improve access to healthcare solutions.
Looking ahead, the future of CROs is marked by a commitment to diversity and inclusivity in clinical trials, ensuring that advancements in treatment are relevant to a broad demographic spectrum. As the industry continues to adapt to new regulations and technological advancements, the collaboration between CROs, sponsors, and regulatory bodies will be paramount in enhancing the quality and compliance of clinical trials. In summary, the indispensable role of CROs not only streamlines the clinical trial process but also contributes to the advancement of healthcare, ultimately benefiting patients and communities worldwide.
Frequently Asked Questions
What are Contract Research Organizations (CROs)?
CROs are specialized collaborators in the pharmaceutical, biotechnology, and medical device industries that provide extensive assistance throughout Contract Research Clinical Trials, including managing operations, executing patient recruitment strategies, and ensuring compliance with regulatory standards.
Why are CROs important in clinical research?
CROs play a crucial role in the effective management of research studies, particularly in addressing the complexities of chronic disease management, which can significantly impact treatment advancements.
How do CROs enhance clinical research in Latin America?
Collaborations like that between bioaccess™ and Caribbean Health Group demonstrate how strategic partnerships with CROs can improve clinical research, with support from local health authorities positioning regions as leading study destinations.
What challenges do US Medtech companies face in Latin America?
US Medtech companies encounter regulatory hurdles, financial constraints, and language barriers that complicate their operations in Latin America.
How are digital technologies impacting CRO operations?
The incorporation of innovative GenAI technologies into research networks aims to improve cooperation, operational efficiency, and data management within the research ecosystem, addressing key challenges faced by Medtech companies.
What services do CROs provide for Contract Research Clinical Trials?
CROs offer a comprehensive suite of services, including feasibility and site selection, clinical study management, regulatory affairs, trial setup and approval, patient recruitment and retention, data management and biostatistics, site management, quality assurance, and reporting.
How do CROs ensure compliance with regulatory standards?
CROs prepare and submit regulatory documents, ensuring adherence to local and international regulations and evaluating their record of regulatory compliance.
What is involved in patient recruitment and retention?
CROs develop effective strategies for recruiting and retaining trial participants, emphasizing the importance of communication to enhance collaboration and compliance throughout the process.
How do CROs manage data in clinical trials?
CROs excel in collecting, managing, and analyzing data using advanced technologies and robust data management systems, ensuring data integrity and supporting evidence-based studies.
What role does quality assurance play in CRO services?
Quality assurance involves implementing rigorous quality control measures to uphold data integrity and ensure compliance with Good Clinical Practice (GCP) standards.
Why is comprehensive reporting important in clinical trials?
Comprehensive reporting on study status, inventory, and adverse events is critical for maintaining transparency and regulatory compliance in clinical research.




