Introduction
As the landscape of clinical research continues to evolve, Contract Research Organizations (CROs) in Latin America have emerged as vital players in the pharmaceutical, biotechnology, and medical device sectors. These organizations not only facilitate clinical trials but also ensure compliance with both local and international regulations, significantly impacting the speed and quality of research outcomes. With a growing demand for clinical trials in the region, particularly in Brazil, the role of CROs becomes increasingly crucial.
They offer a plethora of services, from study design and regulatory affairs to patient recruitment and data management, all tailored to meet the unique needs of Medtech startups and established companies alike. This article delves into the current state of CROs in Latin America, highlighting market trends, key services, challenges faced, and best practices for successful collaboration, ultimately underscoring the importance of these organizations in advancing medical research and improving patient care across the region.
Introduction to Contract Research Organizations in Latin America
Contract Research Organizations (CROs) act as essential enablers within the pharmaceutical, biotechnology, and medical device industries by offering extensive assistance for research projects and initiatives. In South America, the Latin America Contract Research Organization plays an indispensable role in bridging the gap between researchers and regulatory authorities, ensuring that all activities adhere to both local and international standards. Among these, bioaccess® stands out as a leading CRO, renowned for its cost-effective and high-quality services tailored for Medtech startups.
Their partnership with Caribbean Health Group seeks to establish Barranquilla as a leading location for trials in Latin America, backed by a Latin America Contract Research Organization and strong support from Colombia's Minister of Health, Juan Pablo Uribe. The market in this area is anticipated to undergo substantial expansion, especially in Brazil, which is forecasted to exhibit the highest compound annual growth rate (CAGR) from 2024 to 2030, propelled by government reforms that boost pharmaceutical product demand and increasing investments in development. CROs provide a broad range of services, including:
- Study design
- Regulatory approval
- Site activation
- Patient recruitment
- Data management
- Regulatory Affairs
Significantly, bioaccess® has collaborated with GlobalCare Clinical Trials to improve trial ambulatory services in Colombia, achieving over a 50% reduction in subject recruitment time and a retention rate exceeding 95%. This multifaceted support is particularly vital as the Latin America Contract Research Organization market evolves. By utilizing regional knowledge and resources, CROs not only enhance the efficiency and effectiveness of studies but also contribute significantly to the advancement of medical understanding and the improvement of patient outcomes.
As highlighted by Anil Kumar P., a Research Manager in Healthcare, 'Whether collaborating with small start-ups or large enterprises, I consistently provide high-quality analysis that supports informed decision-making and drives impactful outcomes in the healthcare industry.' This quote highlights the reliability of CROs and their vital role in improving study efficiency and overall quality in South America. Furthermore, insights from the case study titled 'Market Segmentation Insights' reveal that the in vivo CRO market is segmented by modality type, including small molecules and various therapies, which highlights the diverse applications and needs within the market.
This segmentation offers a comprehensive insight into revenue expansion across various sub-segments from 2018 to 2030, emphasizing the significance of CROs in managing the intricacies of research.
Market Trends and Growth Forecast for Latin American CRO Services
The Latin America Contract Research Organization (CRO) market has experienced significant expansion in recent years, mainly driven by increasing investments in medical studies and a growing need for localized knowledge. Recent projections indicate that this sector is set to expand at a compound annual growth rate (CAGR) of approximately 10% over the next five years, with Brazil expected to register the highest CAGR from 2024 to 2030. This growth trajectory is supported by several key factors, including:
- A rise in research activities
- Favorable regulatory reforms
- An enhanced focus on patient-centric research methodologies
Moreover, as pharmaceutical firms seek to broaden their research locations, they find South America to be an attractive choice due to its varied patient population and economical operational structures, making it ideal for a Latin America Contract Research Organization. Notably, small molecules accounted for a significant revenue share of 63.66% in 2023, highlighting the importance of this segment within the market. The South American in vivo CRO market, segmented according to small and large molecules, has demonstrated promising revenue growth, particularly from 2018 to 2030.
The region's robust R&D investments and a significant rise in preclinical testing underscore its potential. Government reforms are anticipated to further stimulate demand for pharmaceutical products, while the outsourcing of preclinical services by pharmaceutical organizations is expected to contribute to lucrative market growth. However, Medtech firms in South America encounter obstacles like:
- Regulatory hurdles
- Language barriers
- Restricted access to resources
These challenges can hinder their capability to perform research effectively.
In this evolving landscape, bioaccess® stands at the forefront of Medtech research as a Latin America Contract Research Organization, offering comprehensive study management services that include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
These services not only facilitate smoother legal processes but also contribute to local economies by creating jobs and improving healthcare access. The partnership between Greenlight Guru and bioaccess™ illustrates the dedication to improving the quality of life by speeding up Medtech innovations and trials throughout the region.
As Anil Kumar P., Research Manager - Healthcare, aptly states, 'Whether collaborating with small start-ups or large enterprises, I consistently provide high-quality analysis that supports informed decision-making and drives impactful results in the healthcare industry.' Understanding these dynamics is essential for researchers aiming to collaborate effectively with cross and leverage their services for successful study execution.
Key Services Offered by Latin American CROs
Latin America Contract Research Organization (CRO) in Colombia offers a wide range of services that meet the specialized needs of research for first-in-human medical devices. These services encompass:
-
Study Management: Providing thorough supervision of studies from initiation to completion, ensuring compliance with regulatory standards and improving study efficiency.
Colombia's regulatory review process is notably speedy, with IRB and MoH approvals typically completed within 90-120 days.
-
Site Management: Focused on identifying and managing trial sites, these organizations facilitate access to appropriate patient populations—critical for diverse and effective trials. With a population exceeding 50 million and 95% covered by universal healthcare, Colombia boasts a significant pool of potential participants.
-
Patient Recruitment: Implementing tailored strategies to effectively recruit and retain participants for research studies, addressing the unique challenges presented by diverse demographics. This effort is crucial given that approximately 65% of revenue in this sector is derived from competitive and market intelligence efforts, underscoring the financial significance of effective patient recruitment strategies.
-
Data Management and Biostatistics: Involves meticulous collection, analysis, and interpretation of clinical data, ensuring accurate reporting and compliance with regulatory requirements.
-
Regulatory Affairs: Offering expert advice through the complex regulatory environment, these services guarantee that all studies adhere to local regulations and international standards, thus reducing delays and barriers. Colombia's healthcare system is ranked among the best globally, further enhancing its appeal as a testing destination. Furthermore, the R&D tax incentives offered in Colombia, including 100% tax deductions and various financial advantages for investments in science and technology, significantly attract medical device companies to perform their evaluations here.
-
Pharmacovigilance: Observing and evaluating the safety of pharmaceutical products throughout the research process, which is essential for ensuring participant safety and regulatory adherence.
These services are important for researchers seeking to enhance research outcomes amidst a swiftly changing environment. The impact of Medtech research studies on local economies is also significant, creating jobs and promoting economic growth while enhancing healthcare standards. As emphasized by Oracle Corporation, which has activities in various areas including Latin America, these Latin America Contract Research Organizations play a crucial role in the research ecosystem.
The recent impact of the COVID-19 pandemic has further underscored the necessity of innovative solutions and adaptability in clinical study management. For example, the pandemic has sped up the adoption of remote monitoring technologies and virtual patient engagement strategies, essential for maintaining trial continuity and participant safety, thereby emphasizing the significance of these services in achieving successful outcomes.
Challenges Faced by CROs in Latin America
While the expansion of Contract Research Organizations (CROs) in Latin America presents numerous opportunities, several significant challenges must be navigated to ensure successful research outcomes:
- Regulatory Complexity: The varying regulatory environments across Latin American countries can pose substantial hurdles. The complex and frequently variable regulatory requirements complicate the research process, potentially resulting in delays and higher expenses.
- Cultural Differences: Gaining a deep understanding of local customs and patient expectations is essential for effective patient recruitment and retention. This cultural awareness can significantly impact integrity and participant commitment.
- Limited Infrastructure: In many areas, the absence of sufficient healthcare facilities can hinder the implementation of clinical studies, ultimately impacting the quality of data gathered. This infrastructure gap necessitates strategic planning to ensure experiments can be conducted effectively.
- Competition for Resources: As the demand for CRO services surges, competition for skilled personnel and appropriate study sites intensifies. This competition can result in resource limitations, affecting the overall effectiveness of research studies.
- Economic Factors: Economic instability in the area can directly influence funding for research, restricting the capacity of sponsors to assign essential resources to studies. Fluctuating economic conditions necessitate agile responses from CROs to maintain operational viability.
Addressing these challenges requires proactive communication and collaboration between researchers and the Latin America Contract Research Organization, coupled with a comprehensive understanding of the local landscape. The recent partnership between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, aims to position Barranquilla as a premier location for research studies in South America, emphasizing the importance of a Latin America Contract Research Organization in tackling these challenges. Moreover, as a leading Latin America Contract Research Organization, GlobalCare Clinical Trials has teamed up with bioaccess™ to improve ambulatory services for studies in Colombia, achieving over a 50% decrease in recruitment time and a retention rate surpassing 95%.
As Nigel Unwin aptly observed,
To overlook the noncommunicable diseases would inevitably result in a rise in their burden...
This viewpoint emphasizes the essential need for strong investigation strategies that can adjust to the complexities of clinical trials in South America. Additionally, with 17 comments on the original post, it is clear that there is a growing interest in these topics within the community.
Moreover, the case study titled "Noncommunicable Diseases in Sub-Saharan Africa" underscores the importance of addressing similar health issues in Latin America. The challenges encountered by the Latin America Contract Research Organization in conducting studies on noncommunicable diseases are reflected in both areas, highlighting the necessity for enhanced surveillance and study strategies.
Moreover, the Latin America Contract Research Organization, known as the Asociacion de Cirujanos Traumatológicos en las Americas (ACTUAR), plays a pivotal role in fostering global partnerships aimed at enhancing capacity development in studies. Through various initiatives, ACTUAR aims to tackle these challenges by encouraging cooperation among researchers and healthcare experts throughout the Americas, thus enhancing the infrastructure and resources available for research studies. Significantly, Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared valuable insights about his experience with bioaccess® during its first human trial in Colombia, while Dr. John B. Simpson, CEO of Avinger, Inc., praised Avinger's positive experience conducting OCT-guided atherectomy studies at a facility in Cali, Colombia.
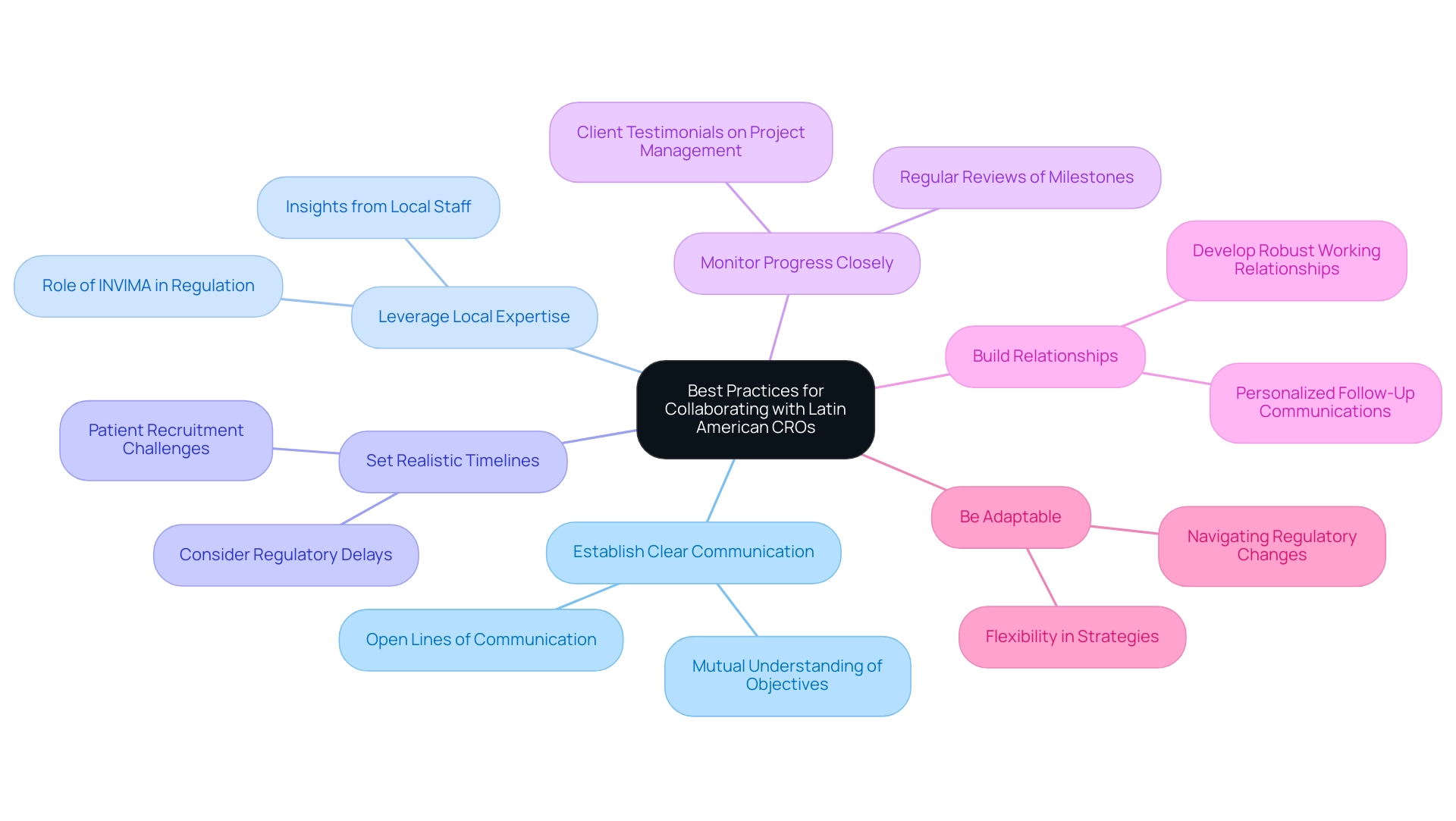
Best Practices for Collaborating with Latin American CROs
To forge effective collaborations with Contract Research Organizations (CROs) in Latin America, researchers should adhere to the following best practices:
- Establish Clear Communication: Initiating open lines of communication from the outset ensures that all parties have a mutual understanding of objectives, timelines, and expectations, paving the way for a successful partnership.
- Leverage Local Expertise: Tapping into the insights and knowledge of local staff in a Latin America Contract Research Organization is crucial for navigating the cultural and regulatory intricacies inherent to the region, thereby enhancing operational efficiency. This is particularly important given the role of organizations like INVIMA, which oversees medical device regulation and ensures compliance with health standards in Colombia, classified as a Level 4 health authority by PAHO/WHO.
- Set Realistic Timelines: It's essential to consider potential delays stemming from regulatory processes, patient recruitment challenges, and local conditions when establishing project timelines to ensure feasibility. For example, Dr. John B. Simpson from Avinger has shared insights on conducting OCT-guided atherectomy studies in Colombia, emphasizing the need for realistic planning.
- Monitor Progress Closely: Regular reviews of project milestones and performance metrics allow for early identification and resolution of issues, which is vital for maintaining project momentum. Client testimonials, such as those from Bill Andrews, Ph.D., President & CEO of Sierra Sciences, underscore the importance of diligent project management in achieving success.
- Build Relationships: Investing time in developing robust working relationships with CRO personnel fosters an environment conducive to collaboration and effective problem-solving. As seen in case studies, personalized follow-up communications can significantly enhance participant retention rates.
- Be Adaptable: Researchers must remain flexible and ready to adjust strategies in response to the dynamic landscape of medical research within the Latin America Contract Research Organization, including regulatory changes and shifting market conditions. Experts such as Katherine Ruiz and Juan Cuya provide invaluable insight in regulatory matters and study management, assisting in navigating these complexities.
Along with these practices, it is essential to acknowledge the extensive services provided by CROss, which encompass feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. Applying these methods not only improves the collaboration experience but also greatly aids in the successful completion of studies. With dropout rates in South America being one-third of those in the U.S. and EU, along with a strong doctor-patient relationship, the region offers a fertile environment for medical studies.
As Julio G. Martinez-Clark, CEO of bioaccess, observes, "The absence of trial awareness, drug shortages, challenging access to the national public healthcare systems, and the strong bond between patients and physicians make Latin America a fertile ground for subject recruitment and retention." Furthermore, Colombia is recognized for its scientific rigor, ethics, and quality of clinical data, enhancing its appeal for clinical research.
Conclusion
The evolution of Contract Research Organizations (CROs) in Latin America marks a significant advancement in the pharmaceutical, biotechnology, and medical device sectors. These organizations are not only crucial for facilitating clinical trials but also play a pivotal role in ensuring compliance with complex regulatory frameworks, thereby enhancing the quality and efficiency of research outcomes. As highlighted, the growing demand for clinical trials, particularly in Brazil, underscores the increasing importance of CROs in navigating the intricacies of the research landscape.
CROs offer a diverse range of services that are essential for the successful execution of clinical trials, including clinical trial management, patient recruitment, data management, and regulatory affairs. The collaboration between organizations such as bioaccess® and Caribbean Health Group exemplifies the potential for these entities to transform regions like Barranquilla into premier destinations for clinical research. With significant investments in research and development, alongside supportive government reforms, the Latin American CRO market is poised for substantial growth.
However, challenges such as regulatory complexity, cultural differences, and limited infrastructure must be addressed to fully realize this potential. By adopting best practices for collaboration—such as clear communication, leveraging local expertise, and maintaining flexibility—researchers can foster effective partnerships with CROs, ultimately leading to successful clinical trials that contribute to improved patient care and medical advancements.
In summary, the role of CROs in Latin America is indispensable in advancing medical research and enhancing healthcare outcomes. As the market continues to grow and evolve, the strategic collaboration between researchers and CROs will be vital in overcoming challenges and maximizing the benefits of clinical trials in the region.
Frequently Asked Questions
What role do Contract Research Organizations (CROs) play in the pharmaceutical and biotechnology industries?
CROs act as essential enablers by providing extensive assistance for research projects and initiatives, ensuring compliance with local and international standards.
How does the Latin America Contract Research Organization contribute to research in South America?
It bridges the gap between researchers and regulatory authorities, facilitating adherence to standards and supporting the expansion of research activities in the region.
What services do CROs typically offer?
CROs provide a broad range of services including study design, regulatory approval, site activation, patient recruitment, data management, and regulatory affairs.
What is the significance of bioaccess® in the Latin America CRO market?
Bioaccess® is recognized for its cost-effective, high-quality services tailored for Medtech startups and has established partnerships to enhance trial locations in Latin America.
What factors are driving the growth of the CRO market in Latin America?
Key growth factors include increased research activities, favorable regulatory reforms, and a focus on patient-centric research methodologies.
What challenges do Medtech firms face in South America?
Challenges include regulatory hurdles, language barriers, and limited access to resources, which can hinder effective research performance.
How does Colombia's regulatory environment benefit CROs?
Colombia has a speedy regulatory review process, with approvals typically completed within 90-120 days, making it an attractive location for conducting trials.
What impact does the COVID-19 pandemic have on clinical study management?
The pandemic has accelerated the adoption of remote monitoring technologies and virtual patient engagement strategies, which are essential for maintaining trial continuity and participant safety.
What best practices should researchers follow when collaborating with CROs in Latin America?
Researchers should establish clear communication, leverage local expertise, set realistic timelines, monitor progress closely, build relationships, and be adaptable to changes.
Why is Colombia considered a fertile ground for medical studies?
Colombia offers a diverse patient population, strong doctor-patient relationships, and recognized scientific rigor, making it an ideal location for subject recruitment and retention in clinical trials.

