Introduction
In the realm of clinical research, the ability to effectively conduct literature searches is paramount for informing evidence-based practice. Clinical evaluation literature searches are systematic processes that not only identify and synthesize relevant studies but also serve as the backbone for regulatory submissions and clinical guidelines.
As the landscape of medical literature continues to expand, understanding the intricacies of these searches—including the types of literature available, the selection of appropriate databases, and the methodologies for organizing findings—becomes increasingly crucial.
This article delves into the essential steps for conducting thorough literature searches, the challenges researchers may face, and the best practices for ensuring a comprehensive and reliable evidence base that supports clinical decision-making.
Understanding Clinical Evaluation Literature Searches
Evaluation documentation inquiries are systematic methods aimed at identifying, analyzing, and synthesizing relevant research studies that are essential in guiding assessments, particularly through a clinical evaluation literature search. The clinical evaluation literature search plays a critical role in gathering robust evidence that supports regulatory submissions, medical guidelines, and treatment protocols. It is crucial to comprehend the different kinds of texts involved—such as peer-reviewed articles, trial reports, and meta-analyses—each adding uniquely to the overall body of evidence.
For instance, the effectiveness of mammography screening is emphasized by the statistic that the number needed to screen (NNS) was 961 over 16 years to prevent one breast cancer death, illustrating the weight of evidence in medical evaluation. Moreover, the total of included and excluded articles should match the overall count of articles acquired from the research, emphasizing the systematic nature of these processes. Systematic reviews are vital for minimizing bias and ensuring comprehensive coverage of relevant studies, which directly influences the quality and reliability of the clinical evaluation literature search.
A pertinent example is the case study titled 'Duct Tape for Treating Common Warts,' which evaluated the effectiveness of duct tape compared to cryotherapy in treating warts among children and young adults. The study found duct tape to be 42% more effective than cryotherapy, although the small sample size of 51 patients raises questions about the reliability of these statistics. As Ron Wasserstein, executive director of the American Statistical Association, stated,
the value was never meant to substitute the scientific reasoning, which is of greater interest.
This viewpoint highlights the significance of organized research in establishing a strong basis for evaluations, ultimately resulting in more informed decision-making in medical practice.
Step-by-Step Guide to Conducting a Literature Search
-
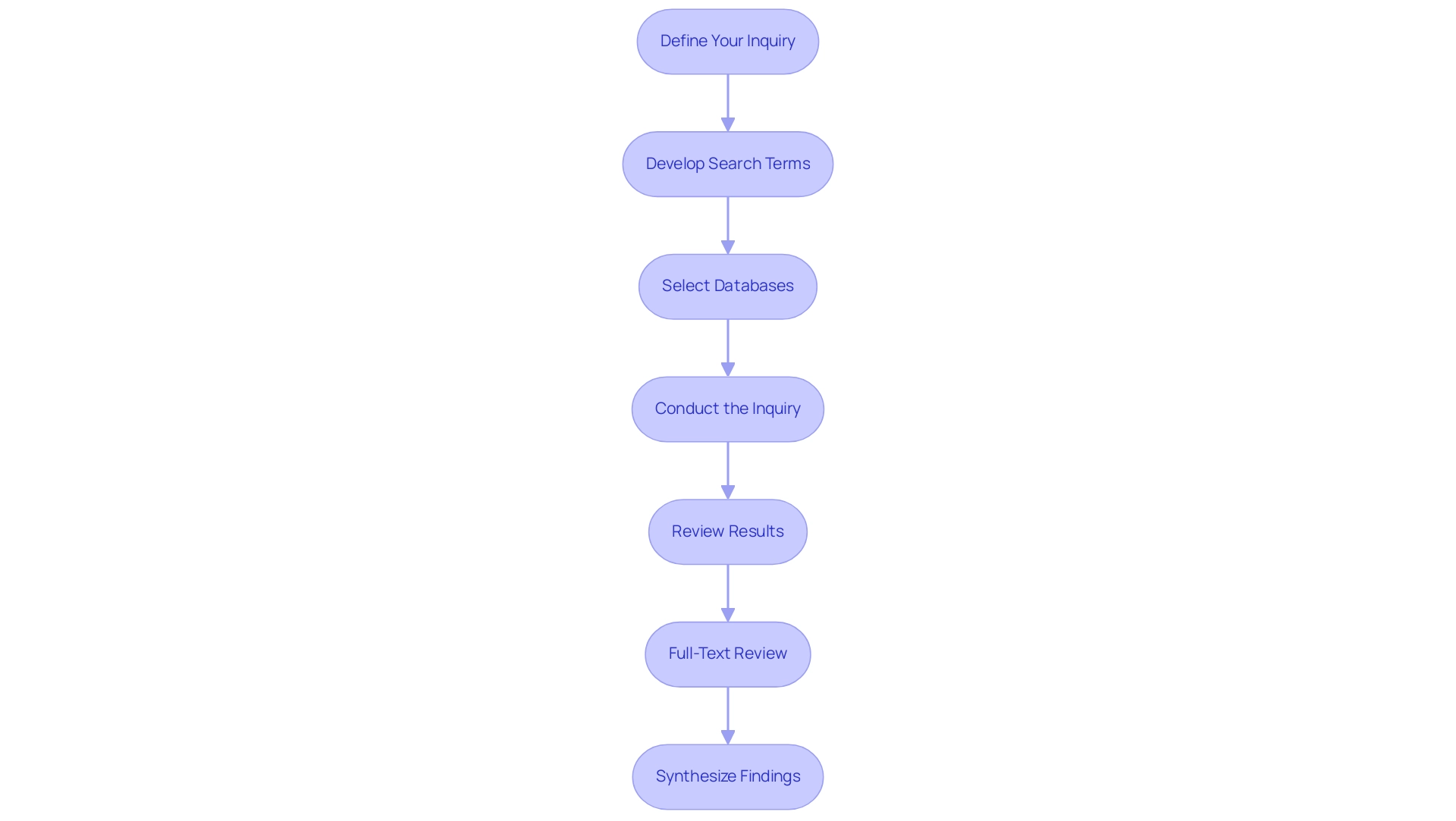
Define Your Inquiry: Start by expressing your medical inquiry with precision. This essential step is vital as it guides your criteria and ensures that your resource exploration is targeted and pertinent. As Anju Grewal, a notable person in clinical studies, states,
The clinical evaluation literature search can be exhaustive and time-consuming, but there are some simple steps which can help you plan and manage the process.
-
Develop Search Terms: Identify a set of keywords and phrases relevant to your inquiry. Additionally, consider employing synonyms and related terms to broaden the scope of your inquiry, facilitating a more comprehensive review of the literature.
-
Select Databases: Choose databases that are specifically tailored to clinical research, such as PubMed, Cochrane Library, Scopus, and Google Scholar. Employing these specialized resources can improve the quality of your findings and enhance access to clinical evaluation literature search.
-
Conduct the Inquiry: Utilize your identified keywords to perform queries within the chosen databases. Incorporate Boolean operators (AND, OR, NOT) to refine and narrow your results effectively. Recent guidelines, including those from PRISMA-S, underscore the significance of conducting a clinical evaluation literature search in systematic reviews, which can greatly improve the effectiveness of this process. In fact, studies have shown that following these guidelines can lead to a more reliable synthesis of findings.
-
Review Results: Quickly skim through the titles and abstracts of the search results to pinpoint relevant studies. Keep meticulous records of citations to facilitate future reference and ensure that you can easily access pertinent literature.
-
Full-Text Review: Retrieve and thoroughly read the full texts of the articles you have identified as relevant. This evaluation is essential for assessing the quality and applicability of the studies to your inquiry.
-
Synthesize Findings: Summarize the essential discoveries from the texts, identifying any gaps in knowledge or conflicting evidence that may require further exploration. Comprehending the interaction between risk management and post-market surveillance in evaluation, as shown in case studies, offers essential insights for your study and improves the overall safety and effectiveness of medical devices.
Choosing the Right Databases and Tools for Your Search
When choosing databases for your medical publications inquiry, it is essential to consider their specific strengths and applications:
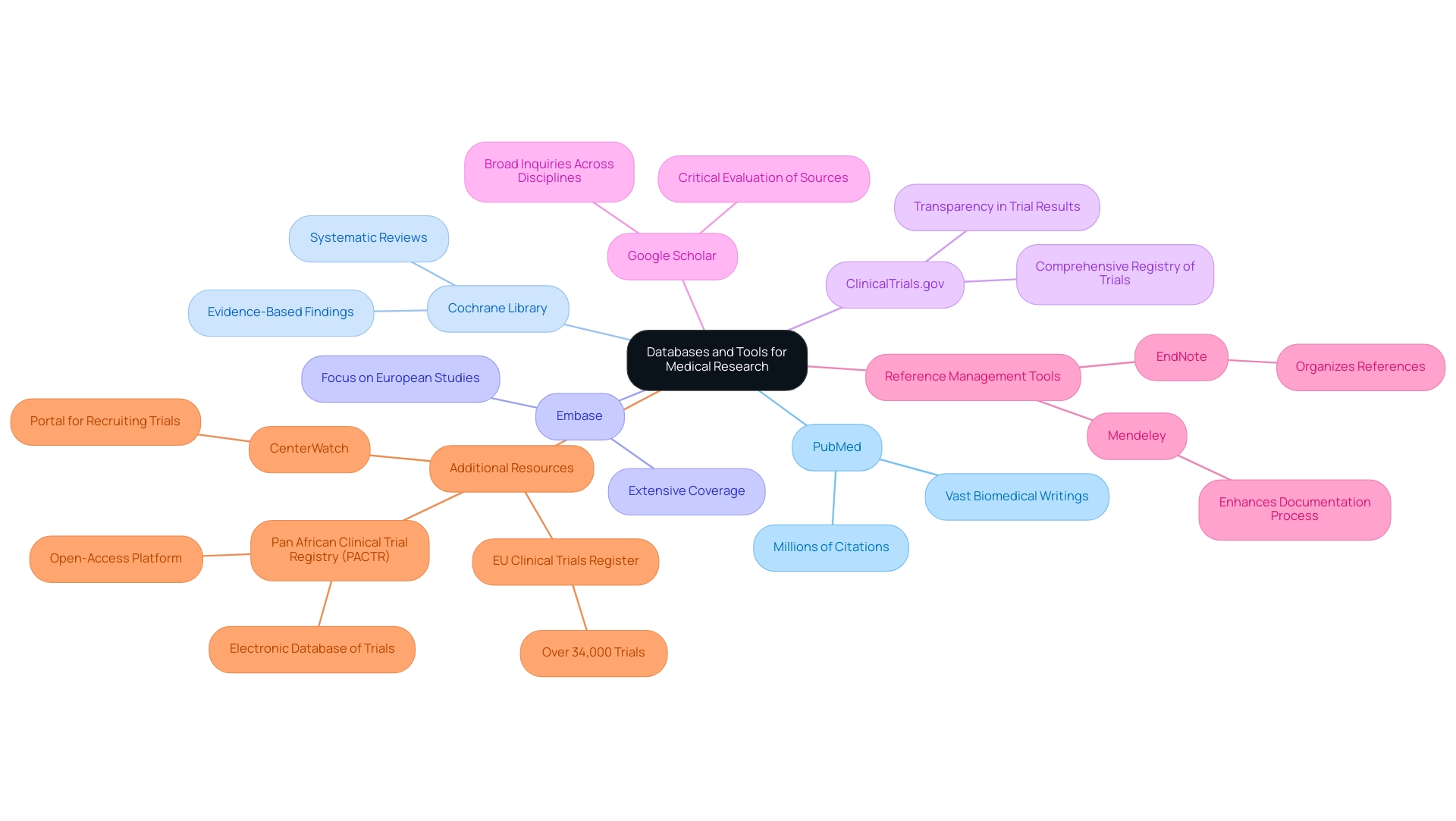
- PubMed: This database is a cornerstone for accessing a vast array of biomedical writings and studies, boasting millions of citations that are invaluable for medical investigations.
- Cochrane Library: Renowned for its systematic reviews, the Cochrane Library provides high-quality, evidence-based findings that are essential for making informed medical decisions.
- Embase: Offering extensive coverage of biomedical documents, Embase is particularly beneficial for scholars focusing on European studies, as it includes resources not found in other databases.
- ClinicalTrials.gov: As a comprehensive registry of trials, this resource is indispensable for locating registered studies and their outcomes, aiding in transparency and the dissemination of trial results.
- Google Scholar: While useful for broad inquiries across disciplines, it is important to critically evaluate the credibility of the sources retrieved from Google Scholar, as not all results meet rigorous academic standards.
In addition to selecting the appropriate databases, utilizing reference management tools such as EndNote or Mendeley can significantly enhance the organization of your references and citations. These tools streamline the documentation process, enhancing overall efficiency in managing your materials. Moreover, as of 2024, the EU Trials Register displays more than 34,000 trials, emphasizing the abundance of information accessible for literature inquiries.
Notably, permalink options are available for sharing search results, which can facilitate collaboration and information dissemination among researchers.
Additionally, platforms like the Pan African Clinical Trial Registry (PACTR) offer free registration for trials, providing an electronic database that supports ongoing research initiatives in Africa. As mentioned by PACTR, it acts as an open-access platform where research trials can be registered free of charge, offering an electronic database of planned trials and trials currently underway. Moreover, CenterWatch serves as a portal for actively recruiting pharmaceutical industry-sponsored research trials, providing a current perspective on available trial databases.
This varied method of database selection guarantees an extensive review of sources, which includes a clinical evaluation literature search, adhering to optimal standards in medical studies.
Organizing and Documenting Your Literature Search
Effectively organizing and documenting your clinical evaluation literature search is crucial for the integrity of clinical research. Here are key best practices to follow:
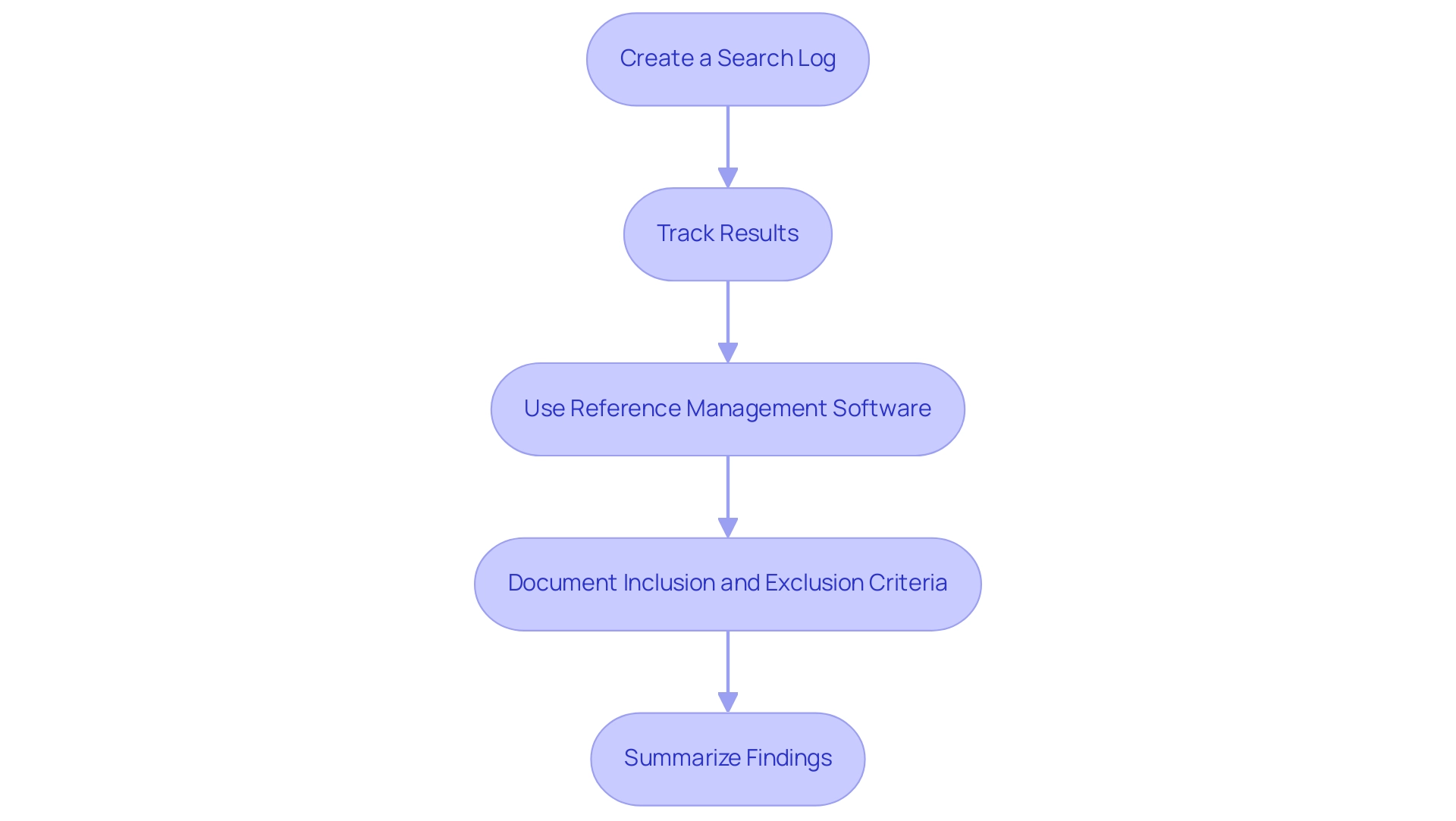
- Create a Search Log: Maintain a comprehensive record of each inquiry conducted. This log should include the databases utilized, specific terms used, and the dates of inquiries. This practice not only enhances transparency but also facilitates easier replication of clinical evaluation literature searches in future studies.
- Track Results: Implement a structured approach by maintaining a spreadsheet or database of the articles reviewed. It is essential to annotate each entry with notes on relevance and methodology quality, facilitating a more efficient analysis and comparison of findings.
- Use Reference Management Software: Leverage tools like EndNote or Zotero to streamline the organization of citations. Recent statistics indicate a surge in the use of reference management software among researchers, highlighting its growing importance in effective documentation. These platforms can simplify the compilation of references when preparing reports or publications, ensuring that all sources are accurately cited and easily accessible.
- Document Inclusion and Exclusion Criteria: Clearly define the criteria used for determining the inclusion or exclusion of studies. This documentation is vital for justifying the selection process and enhances the methodological rigor of your study.
- Summarize Findings: Create a summary document that encapsulates key findings, methodologies, and conclusions drawn from the reviewed materials. This document will act as a crucial reference for ongoing evaluations and can greatly assist in supporting future projects.
These methods not only enhance the effectiveness of information retrieval but also strengthen the overall quality of clinical studies through a clinical evaluation literature search. According to expert Laxmaiah Manchikanti, 'We have developed a new comprehensive instrument to assess the methodological quality of randomized trials of interventional techniques,' emphasizing the necessity for robust assessment tools in studies. Additionally, the case study titled "Key Concepts for Searching Evidence: An Introduction for Healthcare Professionals" demonstrates practical applications of these best practices, outlining essential components for creating a resource retrieval strategy in healthcare.
Optimizing documentation processes is paramount for achieving high methodological quality.
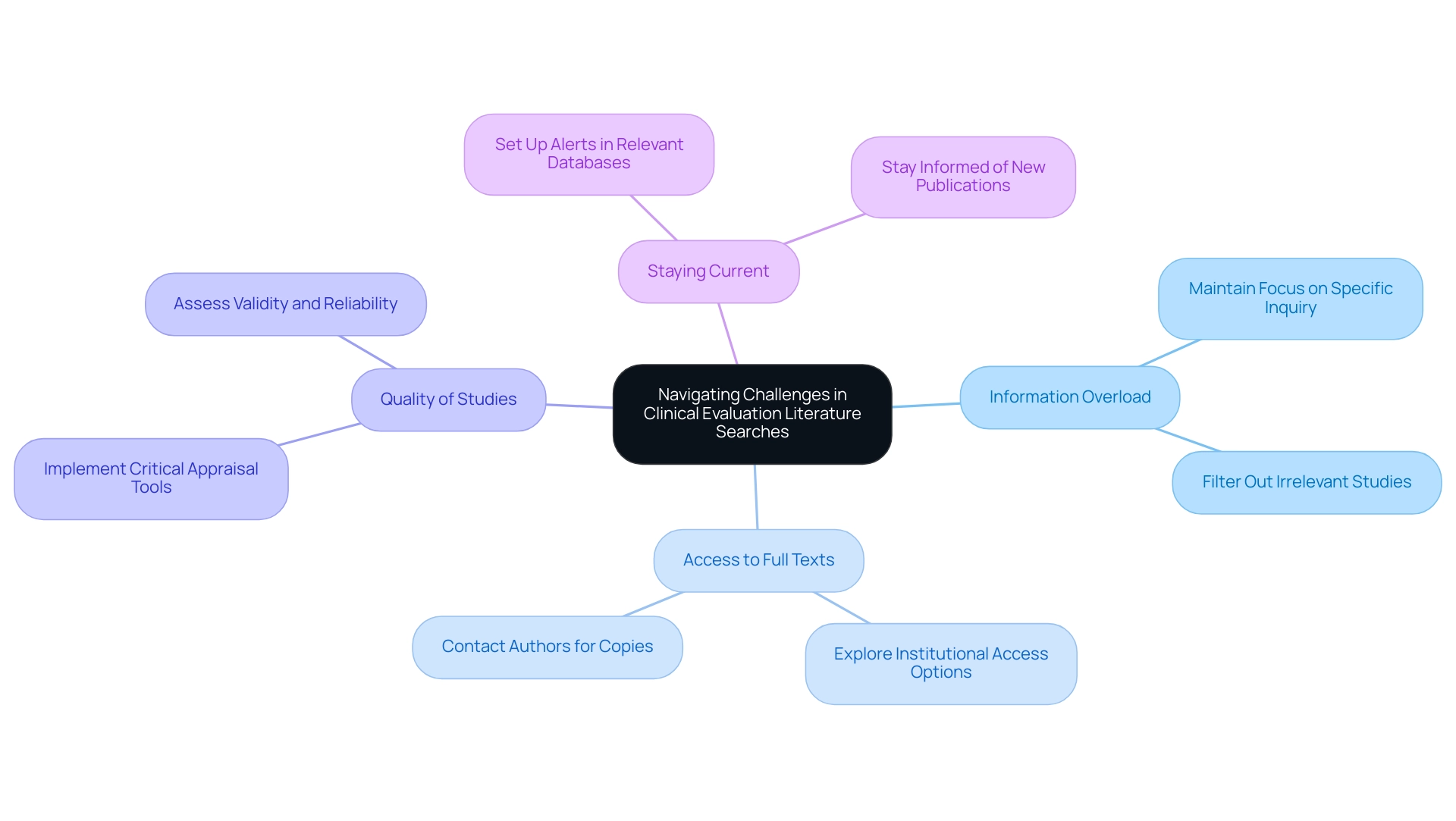
Navigating Challenges in Clinical Evaluation Literature Searches
Clinical evaluation database searches pose several significant challenges that can hinder the investigation process. One significant issue is Information Overload; the sheer volume of available literature can be daunting. To navigate this, it is essential to maintain a clear focus on your specific inquiry, allowing you to filter out studies that do not align with your objectives.
For instance, understanding the effectiveness of different treatments can be illustrated by the statistic that duct tape is 25 percent more effective than cryotherapy in treating warts, showing how statistical evidence can inform clinical evaluations. The problem of Access to Full Texts also arises, as many articles are often concealed behind paywalls. Researchers should explore institutional access options or directly contact authors to obtain necessary copies.
Moreover, the Quality of Studies varies considerably, with not all published works meeting high standards. Implementing critical appraisal tools can help assess the validity and reliability of the studies you encounter. As Scott R. Evans, Ph.D., emphasizes, understanding statistical measures is essential for interpreting study outcomes effectively.
Finally, as the area of medical research evolves rapidly, it is essential to stay current. Setting up alerts in relevant databases can ensure you remain informed of new publications pertinent to your area of inquiry. Furthermore, the case study on poor p-value interpretation emphasizes the typical pitfalls in evaluation resource reviews, underscoring the necessity of using confidence intervals to assess effect sizes.
By proactively tackling these challenges, researchers can significantly enhance both the quality and efficiency of their literature searches, ultimately leading to more robust clinical evaluations.
Conclusion
Conducting effective clinical evaluation literature searches is an essential skill that underpins evidence-based practice in healthcare. As explored in this article, the systematic approach to identifying, analyzing, and synthesizing relevant studies is vital for informing clinical evaluations, regulatory submissions, and the development of clinical guidelines. By understanding the types of literature available, selecting appropriate databases, and employing best practices in organization and documentation, researchers can enhance the reliability and comprehensiveness of their findings.
The step-by-step guide provided highlights the importance of:
- Defining research questions
- Utilizing specialized databases
- Maintaining meticulous records throughout the search process
Additionally, the challenges faced—such as information overload, access to full texts, and varying study quality—underscore the necessity of adopting strategic methodologies to navigate the complex landscape of medical literature. Emphasizing these best practices not only aids in the efficiency of literature searches but also reinforces the overall integrity of clinical research.
Ultimately, a thorough literature search serves as the foundation for informed decision-making in clinical practice. By prioritizing systematic reviews and rigorous methodologies, researchers can contribute to a robust evidence base that enhances patient care and advances the field of medicine. As the landscape of clinical research continues to evolve, embracing these principles will be crucial for ensuring that healthcare professionals remain equipped with the most reliable and relevant information available.
Frequently Asked Questions
What are evaluation documentation inquiries?
Evaluation documentation inquiries are systematic methods used to identify, analyze, and synthesize relevant research studies that guide assessments, particularly through a clinical evaluation literature search.
Why is the clinical evaluation literature search important?
The clinical evaluation literature search is crucial for gathering robust evidence that supports regulatory submissions, medical guidelines, and treatment protocols.
What types of texts are involved in the clinical evaluation literature search?
The types of texts include peer-reviewed articles, trial reports, and meta-analyses, each contributing uniquely to the overall body of evidence.
Can you provide an example of evidence in medical evaluation?
An example is the statistic regarding mammography screening, which indicates that the number needed to screen (NNS) was 961 over 16 years to prevent one breast cancer death, highlighting the weight of evidence in medical evaluations.
What is the significance of systematic reviews in clinical evaluation?
Systematic reviews are vital for minimizing bias and ensuring comprehensive coverage of relevant studies, which directly influences the quality and reliability of the clinical evaluation literature search.
Can you give an example of a case study related to clinical evaluations?
The case study titled 'Duct Tape for Treating Common Warts' evaluated the effectiveness of duct tape compared to cryotherapy in treating warts among children and young adults, finding duct tape to be 42% more effective than cryotherapy.
What steps should be taken to conduct a clinical evaluation literature search?
The steps include defining your inquiry, developing search terms, selecting databases, conducting the inquiry, reviewing results, performing a full-text review, and synthesizing findings.
How can I define my inquiry effectively?
Start by expressing your medical inquiry with precision, which guides your criteria and ensures your resource exploration is targeted and pertinent.
What databases are recommended for clinical research?
Recommended databases include PubMed, Cochrane Library, Scopus, and Google Scholar, as they are tailored to clinical research.
What techniques can improve the effectiveness of a literature search?
Utilizing Boolean operators (AND, OR, NOT) during queries can refine and narrow results, while following recent guidelines like PRISMA-S can enhance the reliability of findings.
What should I do after reviewing search results?
Quickly skim through the titles and abstracts to pinpoint relevant studies and keep meticulous records of citations for future reference.
Why is synthesizing findings important?
Synthesizing findings helps summarize essential discoveries, identify gaps in knowledge, and improve the overall safety and effectiveness of medical devices through better understanding of risk management and post-market surveillance.

