Introduction
In the realm of medical device development, usability testing emerges as an indispensable element, bridging the gap between innovation and user safety. As healthcare technology advances, the imperative to ensure that devices are intuitive and effective for end-users becomes increasingly critical. This process not only identifies potential user errors but also aligns with stringent regulatory standards set by authorities such as INVIMA in Colombia. By focusing on usability, developers can enhance patient outcomes and user satisfaction, ultimately leading to a more reliable healthcare ecosystem.
The following sections delve into the methodologies, standards, and strategic approaches that underpin effective usability testing, illustrating its profound impact on regulatory compliance and market readiness.
The Importance of Usability Testing in Medical Device Development
Usability testing of medical devices serves as a crucial foundation in the development of medical tools, examining how individuals interact with products in practical environments. This usability testing of medical devices is instrumental in identifying functionality flaws that may lead to user errors while also ensuring compliance with essential regulatory standards established by organizations such as INVIMA, the Colombia National Food and Drug Surveillance Institute. INVIMA, designated as a Level 4 health authority by the Pan American Health Organization/World Health Organization, plays a vital role in the supervision of medical equipment, including their functionality and performance.
The Directorate for Medical Instruments and other Technologies within INVIMA is responsible for monitoring and controlling medical instruments, suggesting technical standards, and ensuring compliance with safety and efficacy requirements. By highlighting practical evaluation, developers can significantly improve the safety and effectiveness of their products, ultimately resulting in better patient outcomes and increased satisfaction among consumers. Essential metrics for user experience, such as:
- Task success rate
- Completion time
- User satisfaction
are vital in evaluating the effectiveness of medical equipment.
Moreover, regulatory bodies, such as INVIMA, are increasingly requiring tangible proof of usability testing of medical devices within the submission process, emphasizing its essential function as a key element of device development. As the Human Factors and Usability Engineering Services Market is expected to expand at a 6.2% CAGR from 2024 to 2031, it is clear that the emphasis on user assessment will only increase, requiring that producers adjust accordingly. Furthermore, the expense of tools such as the Excel Pivot Dashboard, costing $1200, emphasizes the investment required in user evaluation procedures.
A recent study supported the implementation of matrix-based models in regulatory standards, offering a structured framework for evaluation that can enhance decision-making for both regulators, such as INVIMA, and manufacturers.

Key Methodologies and Standards for Effective Usability Testing
The assessment of medical equipment is essentially directed by recognized guidelines, with the usability testing of medical devices being foundational in appraising equipment user-friendliness. This standard not only outlines the evaluation process but also conforms to FDA guidelines that emphasize the essential role of usability testing of medical devices in the design and development phases. Effective user experience evaluation involves essential methodologies such as usability testing of medical devices, including formative and summative assessments.
- Formative assessments enable iterative enhancements throughout the development cycle, ensuring that feedback from individuals informs design improvements.
- Conversely, summative evaluation measures the usability testing of medical devices, confirming their functionality, safety, and effectiveness for end-users.
Significantly, the FDA recommends that for products aimed at extensive audiences, a minimum of 30 individuals per group is optimal to guarantee thorough data gathering, highlighting the significance of extensive participant recruitment.
Furthermore, Jakob Nielsen, a researcher at Sun Microsystems, emphasizes the value of iterative testing, stating, 'It is worth noting that Nielsen does not advocate stopping after a single test with five individuals; his point is that testing with five individuals, fixing the problems they uncover, and then testing the revised site with five different individuals is a better use of limited resources than running a single usability test with 10 individuals.'
Furthermore, the case study titled 'Goals of Summative Evaluation' emphasizes the complexities of demonstrating the usability testing of medical devices to prove that a medical device is safe to use regarding its interface. Solutions to these challenges include permitting individuals to repeat procedures and utilizing retrospective data combined with expert assessments during usability testing of medical devices.
Recent insights from industry experts like Tom Rish further reinforce the necessity of addressing human factors throughout the development process to enhance usability testing of medical devices. By following these standards and methodologies, organizations can ensure that their testing is thorough, compliant, and ultimately beneficial for both clients and stakeholders.
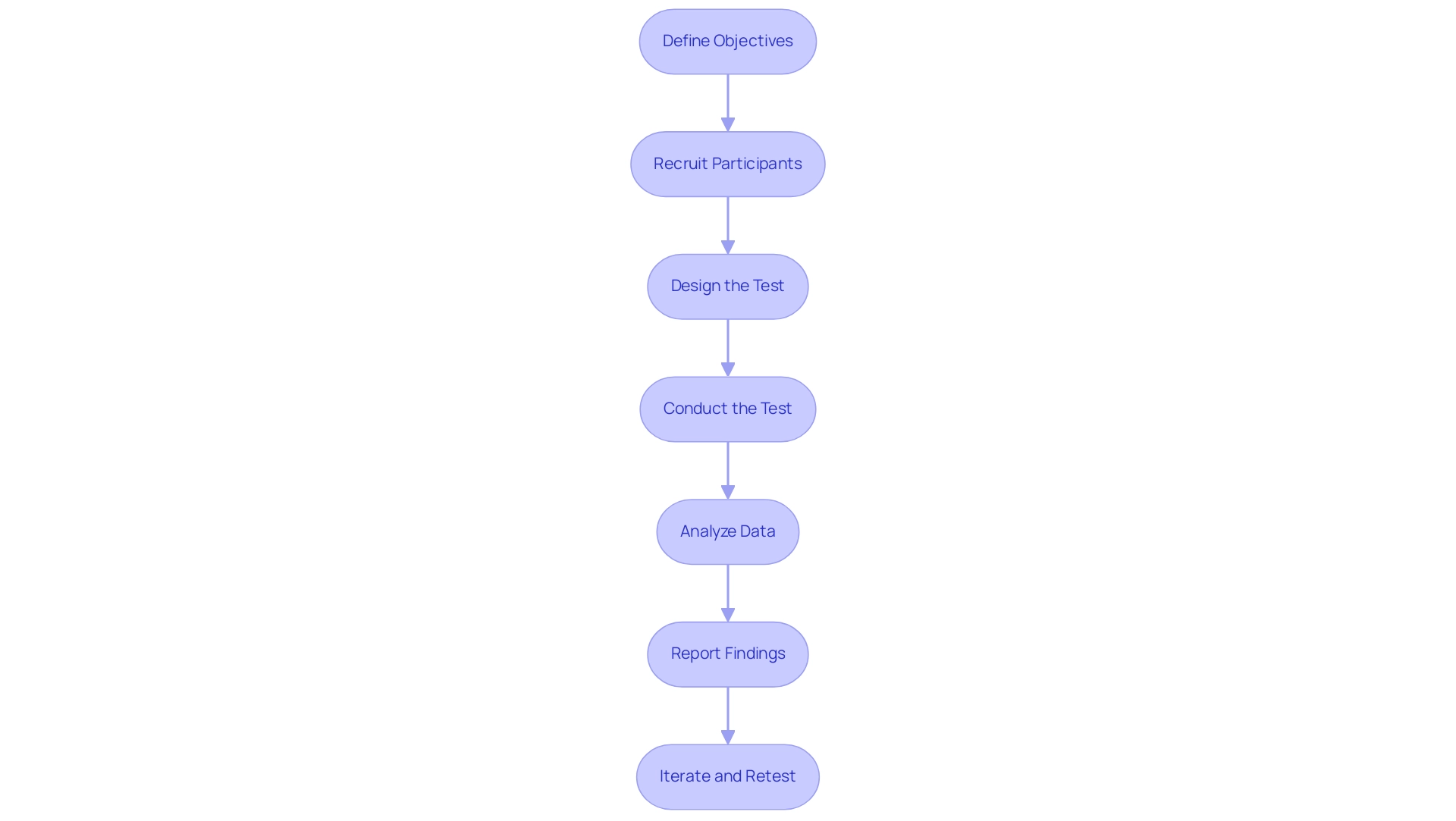
Step-by-Step Process for Conducting Usability Tests
- Define Objectives: Begin by articulating the specific goals of your usability test. Concentrate on the interactions and outcomes you aim to assess during the usability testing of medical devices, ensuring that they align with both regulatory requirements and the needs of individuals.
- Recruit Participants: Carefully identify and recruit participants who are representative of the intended demographic. A diverse sample is crucial for capturing a comprehensive range of user experiences. Notably, qualitative user experience studies typically require 5–10 participants, as indicated by prevailing research practices. However, there is ongoing debate within the research community regarding the ideal number of participants, influenced by factors such as product type, life cycle stage, and researcher skill. Therefore, it is essential to consider your specific context when determining participant numbers for usability testing of medical devices.
- Design the Test: Develop a meticulous test plan that outlines the tasks participants will perform, the testing environment, and the success metrics. This structured approach helps in capturing relevant data and ensures consistency across test sessions in usability testing of medical devices. As emphasized by Marek Štrba, embracing a distinctive UX research approach is vital—there is no universal solution, and comprehending the specific requirements of your audience is crucial.
- Conduct the Test: Facilitate the effectiveness test by observing participants as they engage with the device. Take detailed notes on their interactions during the usability testing of medical devices, noting any difficulties or successes that they encounter. This observational data is invaluable for understanding behavior of individuals.
- Analyze Data: After completing the tests, systematically examine the collected data to uncover user experience issues, emerging patterns, and potential areas for improvement. This analysis should consider factors such as the product type and the life cycle stage, as these can influence the usability testing of medical devices.
- Report Findings: Compile a comprehensive document summarizing the testing outcomes, including actionable recommendations for design modifications based on participant feedback. This report should serve as a guiding document for future development iterations that include usability testing of medical devices.
- Iterate and Retest: Utilize the insights gained from the analysis to implement necessary changes to the apparatus. Conduct further assessments to confirm that the changes have effectively improved the experience of individuals. This iterative process is essential for the usability testing of medical devices to satisfy consumer expectations and regulatory standards.
For further insights on usability testing, refer to Hoa Loranger's checklist for Planning Usability Studies, which provides a succinct overview of key considerations in just five minutes.
Integrating Human Factors into Usability Testing
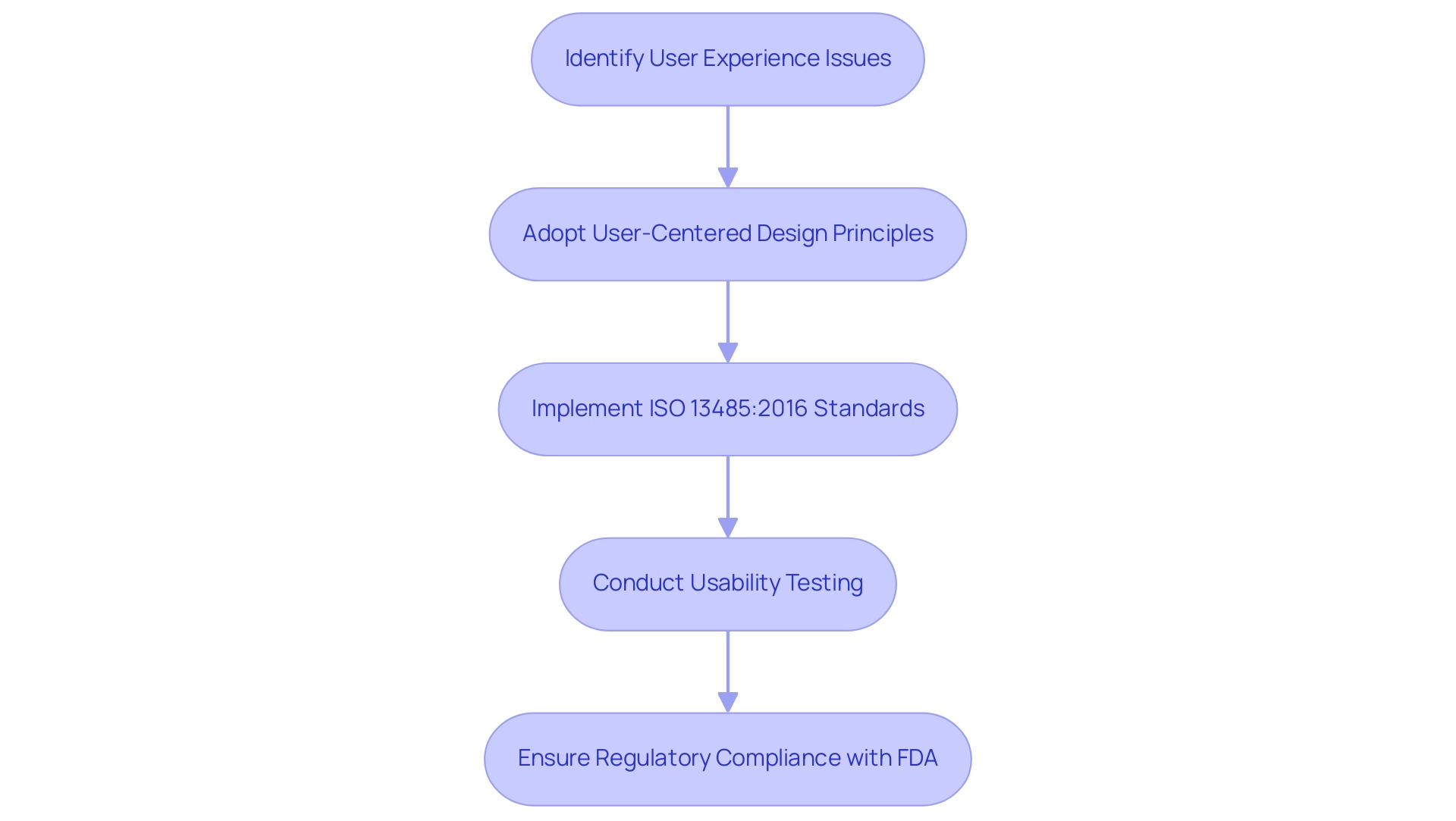
Human elements are essential to evaluating ease of use, as they involve the examination of interactions with medical equipment, concentrating on factors like ergonomics, cognitive load, and overall experience. Medical instruments play an essential role in contemporary healthcare, saving lives and enhancing the quality of life for millions, which emphasizes the importance of usability testing of medical devices. Research indicates that a substantial number of user experience issues in medical equipment stem from design oversights, highlighting the need for a proactive approach.
By integrating human factors into the evaluation process, developers can pinpoint potential problems that may arise, such as confusing interfaces or unclear instructions. This proactive strategy not only reduces risks but also promotes the development of products that are intuitive and user-friendly.
Utilizing user-centered design principles and iterative testing methods greatly improves the functionality of medical equipment. Experts in ergonomics advocate for a design process that prioritizes the needs of individuals, suggesting that such an approach can lead to improved safety and satisfaction. For example, adherence to ISO 13485:2016 highlights functionality requirements, requiring that producers include training for individuals to guarantee the safe and effective operation of equipment.
This case study demonstrates how following these standards assists manufacturers in incorporating user-friendliness into their design processes, ultimately leading to products that better fulfill the needs of healthcare professionals and patients alike. Additionally, user experience engineering seeks to enhance the interaction between individuals and equipment, pinpointing possible user mistakes to reduce risks. As the FDA states,
Except as provided in §§ 56.104 and 56.105, the Food and Drug Administration may decide not to consider in support of an application for a research or marketing permit any data or information that has been derived from a clinical investigation that has not been approved by, and that was not subject to initial and continuing review by, an IRB meeting the requirements of this part.
This regulatory viewpoint emphasizes the essential significance of usability testing of medical devices in the development and approval procedures for medical tools.
Impact of Usability Testing on Regulatory Compliance and Market Readiness
Usability testing of medical devices plays a vital role in achieving regulatory compliance and ensuring market readiness for medical instruments, particularly within the framework established by INVIMA, the national regulatory authority in Colombia. As a Level 4 health authority recognized by PAHO/WHO, INVIMA mandates comprehensive evidence of usability testing of medical devices to validate that they not only meet safety and effectiveness standards but are also easy to use. Successful user experience evaluations can significantly streamline the approval process, reinforcing a manufacturer’s commitment to user-centered design.
Expert perspectives, including insights from Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, indicate that:
- 52% of compliance experts have noted that inadequate information regarding user-friendliness can expose businesses to risks associated with third-party partnerships.
- The Pearson coefficient for the connection between compliance and user evaluation is -0.42, suggesting a negative correlation where insufficient user assessment may relate to heightened compliance difficulties.
On the other hand, inadequately performed usability testing of medical devices can lead to:
- Delays in regulatory approvals
- Increased expenses
- Removal of products from the market
By incorporating thorough user evaluation into the product development process, manufacturers not only improve the overall quality of their devices but also strategically position them within the competitive healthcare environment. Moreover, groups like the Web Accessibility Initiative (WAI), WebAIM, and The A11Y Project offer crucial resources for compliance evaluation, emphasizing the significance of following established standards. Recent insights into user experience assessment methods highlight the significance of human intuition in the evaluation process, underscoring the effectiveness of user-centered approaches.
Furthermore, extensive clinical trial management services, encompassing:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Project management
are essential in aiding evaluation processes and ensuring regulatory compliance. This alignment of usability testing of medical devices with INVIMA's regulatory requirements underscores its vital impact on the market readiness of medical devices.
Conclusion
Usability testing is essential in medical device development, ensuring devices are effective, safe, and user-friendly. This article underscores its importance in identifying user errors and meeting regulatory standards set by authorities like INVIMA. By utilizing established methodologies such as IEC 62366 and employing both formative and summative testing, developers can create devices that align with user needs and regulatory compliance.
The step-by-step process for conducting usability tests highlights the necessity of a structured approach, from defining objectives to iterating based on user feedback. This process not only enhances user experience but also supports continuous improvement, which is critical given the significant impact user interactions have on patient outcomes.
Incorporating human factors into usability testing further strengthens device design. By focusing on ergonomics and cognitive load, developers can proactively address design flaws, resulting in intuitive devices that enhance safety and user satisfaction.
In summary, prioritizing usability testing is crucial for regulatory compliance and market readiness. As the healthcare landscape evolves, a strong emphasis on usability will drive innovations that are both advanced and user-centric. Manufacturers who adopt this focus will enhance product quality and gain a competitive edge, ultimately contributing to a safer and more effective healthcare system.
Frequently Asked Questions
What is the purpose of usability testing for medical devices?
Usability testing of medical devices is crucial for examining how individuals interact with products in practical environments, identifying functionality flaws that may lead to user errors, and ensuring compliance with regulatory standards.
Which regulatory body oversees the usability testing of medical devices in Colombia?
The Colombia National Food and Drug Surveillance Institute (INVIMA) oversees the usability testing of medical devices, ensuring their functionality and performance.
What responsibilities does INVIMA have regarding medical instruments?
INVIMA is responsible for monitoring and controlling medical instruments, suggesting technical standards, and ensuring compliance with safety and efficacy requirements.
What metrics are essential for evaluating the user experience of medical equipment?
Essential metrics include task success rate, completion time, and user satisfaction.
Why is usability testing increasingly required by regulatory bodies like INVIMA?
Regulatory bodies are requiring tangible proof of usability testing to ensure that medical devices are safe, effective, and user-friendly before they can be approved for use.
What is the expected growth rate of the Human Factors and Usability Engineering Services Market from 2024 to 2031?
The market is expected to expand at a 6.2% compound annual growth rate (CAGR).
What are formative and summative assessments in usability testing?
Formative assessments allow for iterative enhancements during the development cycle based on user feedback, while summative evaluations confirm the usability, safety, and effectiveness of medical devices for end-users.
How many individuals does the FDA recommend for usability testing aimed at extensive audiences?
The FDA recommends a minimum of 30 individuals per group for thorough data gathering.
What does Jakob Nielsen suggest about iterative testing in usability evaluation?
Nielsen suggests that iterative testing with small groups is more effective than a single test with a larger group, as it allows for fixing problems and testing revised designs with different individuals.
What challenges are associated with demonstrating the usability of medical devices?
Challenges include proving that a medical device is safe regarding its interface, which can be addressed by allowing individuals to repeat procedures and using retrospective data along with expert assessments.
What insights do industry experts provide regarding human factors in usability testing?
Experts emphasize the importance of addressing human factors throughout the development process to enhance the usability testing of medical devices.




